The biological history of Chlamydia trachomatis is intertwined with the evolution of the man. Infecting Elemental Bodies (EBs), having penetrated mucosal epithelial cells, wrap themselves in a cloak (ĸλαμις) of glycogen that ensures their obligatory intracellular survival and protects this differentiation into Reticulate Bodies (RBs) that feed on cellular ATP. Multiple chemokines and cytokines are involved under the direction of IL-6 in the florid phase and IL-17A in the scar phase. The WHO has successfully identified the SAFE strategy against trachoma (Surgery, Antibiotics, Facial cleansing, Environment) as the blueprint to eliminate the disease by 2020. Recently, interest has been increasingly focused on changing sexual attitudes in different areas of the world, leaving Musca sorbens, Scatophaga stercoraria, and stepsisters fairly blameless, but extolling the role of Chlamydia trachomatis in apparently “sterile” chronic prostatitis or conjunctivitis or, less frequently, in oropharyngitis and proctitis. The addition of an S (SAFE-S) standing for “sexual behavior” was then proposed to also attract the interest and attention not only of Ophthalmologists and Obstetricians/Gynecologists, Urologists/Andrologists, and the School Authorities for information on the prevention of sexually transmitted diseases, but also of Social Physicians and Pediatricians. This means that sexually transmitted infections should be screened in asymptomatic patients with risky sexual behavior or sexual contact with people diagnosed with a transmitted infection.
1. History and Italian Background
There are mentions of trachoma, known since antiquity, in Hammurabi’s Code of the 18th century B.C., also carved into the diorite of the 12th century B.C. in the Sousa stele, now in the Louvre, and in the 16th century B.C. Eber’s Papyrus, now in Leipzig (Figure 1).
Figure 1. Eber papyrus, approximately 1550 BC, Egypt, New Kingdom, XVIII dynasty. Medical papyrus on diseases and herbal knowledge, written in hieratic as a summa of older texts. Line 350. “nekha.t” = this can be interpreted as ‘trachome’, conjunctivitic granulosis; ripple of water = “n” as a phoneme; clumps of papyrus = “kh” as a phoneme, sounds like “ch” in German; vulture bird = “a” as a phoneme (it is generally transliterated with sign “3” to indicate an open “A”); bread loaf = “t” as a phoneme; pustule is a determinative for blister, wound or phlogosis; three vertical lines = indicate a plural form (maybe a number of blisters or tearing). The word “nekha.t” is translated as “an eye disease” [belegt Med. eineAugenkrankheit] in Woerterbuch [the Dictionary]. (Cristiano Daglio. Le Malattie. S. Malgora: Ur Sunu, pp 65–81. Saviolo ed. Vercelli, Italy, 2008. Courtesy Dr. F. Buttiglione PhD and Serena Salmé).
Knowledge of the School of Alexandria is reported in the
De re medica by Celsus, who was active in Rome in the first century AD. In the
Epitome book III
De Trachomate by Paulus Aegineta, in the seventh century A.D., the florid granular aspect of the conjunctiva is described resembling a fig and defined as “sycosis”, while “tylosis” is the chronic form (for treatment: hematite, wine, saffron, Viergespann), but it remains generally confused in the set of conjunctivitis-secreting pathologies [
1]. Francis of Assisi suffered severely on his return from Egypt, where he met Sultan Malik al Kamil in the summer truce at the siege of Damietta in 1219, during the Fifth Crusade. Infected and back in Italy, he was treated with eyelid burns with red-hot iron, not complaining but praising God for the sufferings: he died at 44, almost blind.
Mackenzie [
2] in 1854 wondered if the disease “
est susceptible de se propager par infection, c’est à dire si les miasmes qui s’echappent des yeux de ceux qui en son affecés peuvent, en se repandant dans l’atmosphère, provoquer le développement de la meme affection dans d’autres yeux”, while citing spreads in communities, barracks, schools and families and the possible transmission with infected towels.
Patients were treated both as outpatient or inpatient in general surgeries or the medical hospital, together with syphilodermopathic patients, as in the Ophthalmic Hospital of Turin, Italy, founded by senator Prof. Casimiro Sperino, transforming in 1835, on behalf of Carlo Alberto King of Sardinia [
3], the previous hospitals (the so called ‘lazzaretto’: pesthouse) laid for the cholera epidemic.
There, of the 12,114 patients hospitalized in the decade 1873–1883 in the Ophthalmology Clinic of the R. University of Turin headed by Prof. Carlo Reymond, Dr. Camillo Gallenga published 1.392 cases of corneal leucoma including granular conjunctivitis, phlyctenular and pustular affections of the conjunctiva, ulcers and corneal abscesses “
of which often represent the final outcome” [
4], treating with Snellen’s procedure the entropion with trichiasis in severe forms of granular conjunctivitis [
5].
Trachoma has been classified with etiological/microbiological identity in Ophthalmology treatises since the second World War, when the basophilic bodies of Halberstaedter (German dermatologist and radiologist) and von Prowazek (Austrian biologist/zoologist), identified in 1907 in Jakarta during a scientific expedition to study syphilis transmission [
6], were definitively attributed to
Chlamydia trachomatis (C
t). Halberstaedter and von Prowazek, in an expedition to Java, also infected orangutans with conjunctival scrapings from trachoma patients and such agents in conjunctival smears. They called them “chlamydozoa”.
Just two years later, in 1909, an early confirmation was published by Camillo Gallenga, actually Professor of Ophthalmology in the University of Parma [
7], replicated the next year 1910 for the German literature in
Klinische Monatsblatter Augenheilkunde [
8], contrasting opposite skeptical opinions largely represented by Scientists and Ophthalmologists not only in Europe.
Axenfeld [
9], in 1914, still attributed the spread of trachoma in Europe to Napoleon’s Egyptian campaign, linked to granulomatous conjunctivitis, whose diagnosis became certain when it was confirmed by “
corpuscules trachomateux de Prowazek” stained with Giemsa, accordingly with the 1907 first description of the etiology, supported by the Italian literature [
7,
8].
The May–Grunwald Giemsa solution stains chromatin in red-purple (Romanowsky effect), nucleoli in deep blue and the cytoplasm of epithelial cells in light blue. The smallest inclusions, even two or four in number, are generally associated in small aggregates with an intensely basophilic matrix and with a homogeneous surface element that differs from the cytoplasm and colors in intense blue, like a cap. Although differentiated, these Reticulated Bodies (RBs) tend to adhere to the nucleus. The initial inclusions are replaced by Elementary Bodies (EBs), which color red-purple and remain well separated from each other [
10]. The amorphous masses of the blue-colored inclusions envelop like a cloak (κλαμίς: the ancient Greek term for the short cloak worn by Greek military men, draped and secured with a brooch on the right shoulder) the purple-red granules of chromatin ‘carriers of the virus’. The Chlamydiae were named this way because the intracytoplasmic inclusions formed by this agent inside host cells cluster around (are ‘draped’ around) the nucleus of the cell [
11]. Still, in 1925, Amilcare Bietti discusses the distinction or unity of trachoma and ophthalmo-blennorrhea by inclusion bodies in children [
12]. He reports—disputing the hypothesis—that Lindner, on the contrary, was convinced that the two affections were identical, although the latter was unable to infect the genitals of a female baboon with human ocular trachomatous material. Riccardo Gallenga, while operating the plasmoma of the superior bulbar conjunctiva of an eighteen-year-old boy, trachomatous from infancy and now in the scarring phase (Trachoma register int. N° 113 18, 1931, Ophthalmology Clinic R. University of Turin, head Prof. Luigi Guglianetti) in the accurate description of the histological examination, reports “
in the remarkably thickened epithelium... the presence of polynuclear leukocytes, lymphocytes and plasmacells”, but “
I have not observed either epithelial cells or trachomatous granules” [
13] and describes, in the plasmome, the presence of “
rare Russel bodies colored in pink with eosin and in yellow with van Gieson”: the reference documents the absence of Chlamydia in the bulbar conjunctiva of trachoma in the MacCallan cicatricial stage T4, and the precise knowledge of the results by Halberstäedter and von Prowazeck.
The public health response to the spread of trachoma throughout the Mediterranean basin in the 19th and first half of the 20th century involved anti-trachoma clinics and the establishment of dispensaries in all major cities and hospitals. In all of them, there was intense activity and attendance. So much so that in the years 1936–1942, before and during the Second World War (but before the RAF and USAF heavily bombed the city, port and hospital of Cagliari, Sardinia), the flow of patients to the anti-trachoma department of the Cagliari University Eye Clinic headed by Professor Riccardo Gallenga was so intense that it had to be regulated every morning by the traffic police. There, in 1938, by suturing the conjunctival biopsy from surgery for entropion by a T4 patient to the mucosa of his own conjunctival fornix, Riccardo Gallenga also demonstrated that cicatricial trachoma is no longer contagious (Lessons, The Ophthalmology Course, 5th year Medical School University of Turin, 1966; and OMC&O Turin conference, University Eye clinic, 1967) [
14], accordingly with his previous results as Assistant Professor in Turin Eye Clinic [
13].
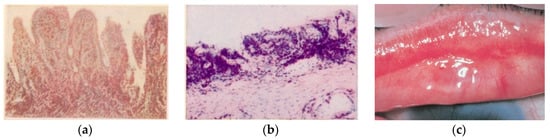
In the second half of the 1950s in Sicily island, another highly involved area, the experiences gathered in the Catania University Eye Clinic, directed by Prof. Giuseppe Favaloro, allowed Giuseppe Scuderi to publish a textbook and atlas of trachoma histopathology [
17], clarifying from a clinical and anatomopathological point of view many aspects related to trachomatous infection (
Figure 2a–c).
Figure 2. (
a) Tarsal conjunctiva. Florid trachoma. (TF, according to WHO classification). Papillary infiltration, forming nodules. Van Gieson ferric hematoxylin. 75×. (
b) Tarsal conjunctiva. Scarring trachoma. From TI (T. Inflammation Intense: fibroblastic proliferation of the chorion, still site of histiolymphocytic infiltration) to TS (T. Scarring): conjunctival scars with fibrous white bands (arrow). Absence of infiltrate and C
t in the scar area. Giemsa 120× [
16], (Courtesy Prof. Nicola Delle Noci). (
c) Tarsal conjunctiva. (MacCallan T4); TS: Arlt’s line. (Reproduced with permission—reference number 230716-012078. From Weisenthal RW.
External Disease and Cornea. Basic and Clinical Science Course, Section 8, American Academy of Ophthalmology, 2013–2014).
2. Contagion and Clinic, The SAFE-S Strategy
In the four clinical stages of trachoma (McCallan, 1908, cited by Frezzotti and Guerra [
38]) from lymphoid hyperplasia to the florid highly contagious granulomatous stage 2—for which ancient treatments with massage, forceps, silver nitrate or copper sulfate brushing have found promoters and innovators over time—the evolution becomes complicated with the upper corneal “neovascular panniculus”. Leaving Herbert’s dimple in place of emptied limbal follicles, with large polynuclear macrophages and Moauro–Leber cells, the evolution of the disease follows the stages of xerophthalmia and tarsal scarring—Arlt’s line—inducing scar entropion, trichiasis and corneal ulcers with a likelihood of superinfection [
4,
7], causing blindness and needing surgery, but which is different from the past (
Figure 4). However, the large burden of symptoms overlapping with other diseases or syndromes, and the priority identification as an exclusively ocular disease, delayed the identification of
Ct by WHO as a sexually transmitted infection until 1976 [
39].
Figure 4. Everting sutures for entropion. George Bartisch: Ophthalmodouleia, 1583. Courtesy Prof. Nicola Delle Noci [
40].
Infection can be caused either directly or indirectly. In the indirect mechanism, flies, in particular, may be involved, which contribute to the spread of the disease by settling on the eyes of infected patients. Contact with eye or nasal secretions (including through cloths or clothing) of infected patients, on the other hand, constitutes a direct route of transmission of the disease.
The spread of the disease is inversely proportional to the socioeconomic development and sanitation conditions; in particular, risk factors can be identified in the “
lack of clean hot and running water, lack of personal cleanliness, crowding, promiscuity, superstitions, and abundance of flies” [
38]. Infection does not confer permanent immunity, so there is a likelihood of re-infection if the environmental causes of infection are not changed. An effective protocol of cyclic therapy protracted for four months to counteract the
Ct biological cycle (EB-RB-EB) was developed by Gallenga PE et al., in 2018, achieving a complete microbiological and clinical resolution, leading to a favorable evolution in some cases of male (caused by chronic prostatitis) and female infertility, thus overcoming the “cultural era” to move to the “molecular era” [
35].
Antimicrobial resistance is a major concern with the potential to cause millions of deaths, even though few studies recorded the clinical resistance of neglected tropical diseases (NTD), namely human African trypanosomiasis, leishmaniasis, onchocerciasis, schistosomiasis, soil-transmitted helminths, and trachoma [
41]. Researches are in the pipeline to find which of the more than 2000 Antimicrobial peptides (AMPs) with broad-spectrum antibacterial, antiviral and antiparasitic activity, that have been shown to be effective against a variety of NTDs including trachoma, could be developed and used as alternatives [
42,
43]. Pep-1, LL-37, and melittin showed outstanding abilities in inhibiting the growth of
Ct. The same applies to gene therapy: IRF5 and IL-10RA are related to macrophage–chlamydia interactions. Their roles seem vital in order to cure
Ct infection in the scenario of the increasing antibiotic resistance, also to azytromycin. However, multiple challenges for the development of these therapies remained [
43].
From the pictures of dirty and tearful children (
Figure 5) in environments with poor sanitation in the Afro-Arabo-Indo-Asian basin, the contagion by flies is evident, although this does not account for the primitive forms of the disease in adults and those outside the tropical–equatorial area [
36]. The WHO [
44,
45,
46] and the Ligue contre le tTrachome (LCT) have rightly and successfully identified the SAFE strategy against trachoma (Surgery, Antibiotics, Facial cleanliness, Environmental improvement) as the blueprint for eliminating the disease by 2020; however, the evidence of the sexually transmitted disease still makes
Musca domestica,
Musca sorbens [
47],
Scathophaga stercoraria [
48,
49] or
Sarcophafaga carnaria, so beloved by fishermen, the culprits.
Figure 5. A Sri Lankan child with eyes infested with flies (M. sorbens). Courtesy Imperial College London. Institute of Global Health Innovation. The impact of Neglected Tropical Diseases on Universal eye health, 2016.
Having this scenario clear, we proposed to complete the acronym SAFE with -S (SAFE-S), understood as ''strategy for controlling sexual well-being, i.e.: sexual behaviour'' [35]. Unprotected sex is guilty: the WHO recomendations for the next decade2021-2030 must attract the interest and attention not only of Obstetricians/Gynecologists, Urologists/Andrologists and Pediatricians, but also High School Authorities that should be mandated to improve students' knowledge on sexually transmitted disease education and prophylaxis/protection.
Conclusions
The oculo-genital correlation and the need for adequate systemic coverage are confirmed by cases of chronic conjunctival disease resolved with ultrasound-guided infiltrative therapy of chronic prostatitis [50] or recurrent atypical chronic oropharyngeal inflammation resolved after chlamydial antibiotic therapy [51].
Therefore, we consider the occasion of this edition useful to relaunch an alert also for the Practitioners and the Social Physicians to holistically evaluate the patient, and in front of multisite infection “check for the presence of typical signs (Arlt’s line [5]; Neri’s white line [52]) or consequences: entropion and pannus, infertility [53], spontaneous abortion, pelvic inflammatory disease” [1,37], cervical cancer in association with HPV, and proctitis especially in homosexual males [33]. Ct also appears to be involved in oculo-orbital lymphoma [54], previously associated only with Chlamydia psittaci. New research would be desirable to confirm or exclude the hypothesis of Chlamydia pneumoniae as a chronic inflammatory stimulus for neovascular Age-related Macular Degeneration (nAMD) [55], inducing the inflammatory state [56]. Thus, it seems appropriate to draw the attention to Chlamydia for an evaluation of florid or chronic conjunctivitis, as well as for oropharyngeal diseases, such as Neisseria gonorrhoeae and other sexually transmitted diseases [57] which could also trigger, sometimes, reactive arthritis [58,59]. The PAHO-WHO plans to control the worldwide trachoma infection by 2020, between 2018 and 2019 showed reduction of 14,4 million people that lived in areas with high trachoma prevalence, but still leaving a total burden of 2,5 million cases of trachomatous trichiasis in 2019. This urgently requires an increase in medical and surgical strategy with decisive support for research and development of an effective and durable vaccine against chlamydial and mycoplasmal infections [60,61], improving the prevention by considering SAFE-S strategy, rather than insect powder.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12121419