Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Gel systems are widely used as plugging materials in the oil and gas industry. Gas channeling can be mitigated by reducing the heterogeneity of the formation and the mobility ratio of CO2 to crude oil. Cracks and other CO2 leaking pathways can be plugged during the geological storage of CO2 to increase the storage stability. By adding CO2-responsive groups to the classic polymer gel’s molecular chain, CO2 responsive gel is able to seal and recognize CO2 in the formation while maintaining the superior performance of traditional polymer gel.
- CCUS
- gas channeling
- CO2 leakage
- CO2 responsive gel
1. Introduction
Gas channeling, as the primary issue that restricts the significant improvement of CO2-EOR, requires an understanding of its generation mechanisms. The factors causing gas channeling can be attributed to the significant physical differences between CO2 and crude oil and reservoir heterogeneities. The former can be divided into two situations (see Figure 1): (1) the gravity overriding phenomenon: during CO2 flooding, due to the low density of CO2, a large amount of CO2 will gradually migrate above the crude oil, ultimately forming a gas channel at the top of the crude oil seepage channel [1]; (2) the viscous fingering phenomenon: the viscosity of CO2 is lower than the viscosity of crude oil, which can cause uneven propulsion during the displacement process. When the flow rate of CO2 in a local area is too fast, gas channeling will occur [2]. The gas channeling caused by reservoir heterogeneity can be divided into the following scenarios: (1) when there are high-permeability channels such as natural fractures, artificial fractures, wormholes, and conduits, CO2 will bypass the matrix and cannot displace the crude oil inside [3][4]; (2) when there is a significant difference in the permeabilities of different layers or zones, CO2 preferentially flows through high-permeability reservoirs [5]; (3) the presence of wormholes, ducts, and high-permeability cracks in the matrix leads to the ineffective flow of CO2 [6].
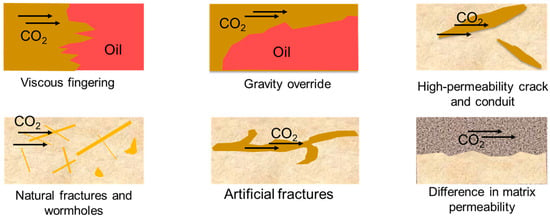
Figure 1. Formation mechanisms of gas channeling.
At present, there are several methods to mitigate oil and gas channeling in CO2 flooding: water-alternating-gas (WAG) injection, the direct thickening of CO2, hydrogel plugging, foam plugging, and nanoparticle plugging. These methods for suppressing CO2 gas migration have been proven to be effective in practical applications and laboratory simulations. The main purpose herein is to figure out the sealing mechanisms of different CO2 channeling control methods and the parameters that affect their effectiveness.
2. Water-Alternating-Gas (WAG) Injection
WAG is considered a reliable method to suppress gas channeling during CO2 flooding [7][8]. Awan et al. reported that, in the WAG process, CO2 flooding provides high sweep efficiency at the micro level, while water flooding provides high sweep efficiency at the macro level. The organic combination of the two results in a significant improvement in WAG oil recovery overall [9]. According to Leeuwenburg’s explanation, the injected water during WAG oil displacement can adjust the CO2 flow to a certain extent. If CO2 is suppressed from flowing to high-permeability zones, it is forced to displace oil left in low-permeability zones, thereby improving the overall oil recovery performance [10]. Kamali et al. also proposed a similar view, using three oil displacement methods: continuous CO2 injection, simultaneous CO2 and water injection, and WAG injection to conduct displacement experiments on sandstone cores. It was found that WAG had the best effect and continuous CO2 injection had the worst effect. Then, numerical simulation experiments were conducted, and it was found that the presence of injected water during the WAG process effectively reduced the permeability of CO2, thereby reducing the mobility ratio of CO2 to crude oil [11]. Han explained the enhanced oil recovery mechanism of WAG from the perspective of miscible displacement, and they believe that an increased volume of injected water significantly increases the injection pressure of CO2, making it easy for CO2 to mix with crude oil. This reduces the interfacial tension between crude oil and CO2, as well as the viscosity of crude oil, allowing more formation crude oil to be extracted from the ground [12].
It is worth noting that precipitation is often generated during the WAG process, which can hinder the flow of crude oil in the formation and affect the improvement of oil recovery. According to the type of precipitation, it can be divided into organic precipitation and inorganic precipitation. Organic precipitation usually refers to asphaltene precipitation [13]. Due to the injection of foreign fluids, changes in the thermodynamic parameters (temperature and pressure) and composition of the crude oil system will lead to aggregation reactions of asphaltene in the crude oil, resulting in solid asphaltene precipitation. The generated asphaltene precipitate will block the channel and seal the reservoir pores, ultimately leading to a decrease in reservoir permeability. Usually, a precipitation inhibitor is added to the injected fluid to hinder the flocculation of unstable asphaltene or make the generated asphaltene precipitate easy to wash out, thereby alleviating the blocking effect of precipitation on the formation. Inorganic precipitation mainly includes metal carbonates. During the process of alternating water and gas injection, some CO2 dissolves in the injected water, converting it into acidic carbonated water and accumulating CO32− in the aqueous solution. When water contains metal scale ions such as Mg2+ and Ca2+, CO32− will combine with metal scale ions. When the concentration of metal carbonate reaches a critical value, precipitation occurs. These sediments can block small pores, leading to a decrease in reservoir permeability. In addition, acidic carbonated water can also erode the reservoir, alter its permeability and pore structure, and cause metal scale ions in the reservoir to enter the injected water, further exacerbating the generation of precipitation [14]. Therefore, for the injected water in WAG, chelating agents should be used before injection into the formation to reduce the content of Ca2+ and Mg2+. Alternatively, inhibitors can be added to inhibit the formation of precipitation.
The recovery efficiency of WAG oil displacement will be greatly impacted by the injection parameters, which include porosity, permeability, and other formation properties, as well as injection parameters like the water to gas slug ratio. Hao et al. first used a thin tube experiment to determine the minimum miscibility pressure of CO2 and crude oil at 22.79 MPa, and then connected three different permeability cores in parallel [15]. After CO2 displacement, it was changed to WAG displacement, and it was found that under both displacement conditions, the core with the highest permeability contributed the most to the recovery performance, both exceeding 90%. The injection pressures were kept at 15 MPa and 25 MPa, respectively, with oil recovery efficiencies of 33.01% and 39.42%, respectively. A higher injection pressure was beneficial for oil recovery improvement [16]. Hosseini and Wang et al. found that, after CO2 and WAG displacement, the oil permeability and porosity of the core significantly decreased, and some areas of the core showed a reversal of wettability. They attributed this phenomenon to the presence of CO2-caused precipitation and the accumulation of asphaltene in the crude oil, blocking some channels, resulting in a decrease in crude oil permeability and core porosity. A combination with surfactants or other types of chemical inhibitors during WAG flooding has been reported to reduce asphaltene precipitation [17][18].
3. Direct Thickening of CO2
The density and viscosity of CO2 gas can be increased by thickening it with polymers, which reduces gas channeling issues brought on by gravity overlap and viscous fingering phenomena [19][20]. Brien believes that the increase in density and viscosity can be achieved by controlling the concentration of the added polymer [21]. Polymers enhance the density and viscosity of CO2 at different levels by dissolving them in CO2. In general, the higher the molecular weight of a polymer, the greater its viscosity, and the better its thickening effect on CO2. However, the higher the molecular weight of the polymer, the lower its solubility in CO2, which is unfavorable for CO2 thickening. Therefore, using low molecular weight polymers to thicken CO2 is also a feasible option. Siloxane polymers have been proven to be an effective CO2 thickener [22]. Bac tested the thickening ability of polydimethylsiloxane (PDMS) on scCO2 at 2500 psi and 130 °F. It was found that the viscosity of CO2 increased from 0.04 cp to 1.2 cp after thickening. In addition, the use of toluene results in the higher solubility of polymers under the same pressure conditions. Bac conducted CO2 core displacement experiments and found that, after adding polymers, the oil recovery rate increased, the gas breakthrough was delayed, and the oil recovery rate increased by 3.4–9% from its original value [23].
There is also a special polymer, which can form a three-dimensional grid structure through a cross-linking reaction between molecular chains, and the network can swell in water. This kind of polymer is called a gel [24][25][26]. Because gels have good plugging properties, they often block high-permeability channels in CO2-EOR, thus inhibiting gas channeling caused by formation heterogeneity [27]. There are two solutions for polymer gels used for CO2 consistency control. The first solution is in situ gel plugging, which injects the solution composed of a polymer monomer, cross-linking agent, and auxiliary agent into the formation to form a gel in the formation and block the migration of CO2 in the high-permeability channel. The second scheme is pre-crosslinked gel plugging, which can be directly injected into the formation after the gel has been completely formed. Alternatively, it can be processed into particles and prepared with a solution, and then injected into the formation [28]. Durucan et al. carried out the core displacement experiment of supercritical CO2 oil displacement, injected polyacrylamide-based polymer gel into the core, and then conducted the CO2 displacement experiment again, and found that the permeability of CO2 decreased by 99% [29].
4. Foam Injection
Foam is a gas dispersion system surrounded by liquid film prepared and stabilized by a surfactant [30][31]. Surfactants are amphiphilic compounds, which means they are composed of hydrophilic heads and hydrophobic tails. They are generally divided into four types (according to the charge of the head group): non-ionic, anionic, cationic, and zwitterionic surfactants. Yan et al. found that foam has greater effective viscosity, which can alleviate gravity overlap and the viscous fingering phenomenon during CO2 flooding, and improve sweep efficiency during CO2 flooding. Foam can also control the local flow resistance of CO2, forcing it into the low-permeability area and displacing the crude oil [32]. Through core displacement studies, Boud and Holbrook demonstrated for the first time that foam may be used to improve oil recovery by gas flooding. Additionally, foam can be produced in reservoir rocks under both miscible and immiscible circumstances using this water-soluble foaming agent. Ren et al. tested the effects of three different types of surfactants on CO2 flooding. The first two surfactants were 2 EH-PO5-EO15 and 2 EH-PO5-EO9, both of which were nonionic surfactants. The third type was the water-soluble anionic surfactant CD-1045. The phase behavior experiments conducted showed that none of these three surfactants could significantly reduce the interfacial tension between water and crude oil. However, all of them can significantly improve crude oil recovery. Compared with pure CO2 flooding, the three surfactants can increase oil recovery by 71%, 92%, and 54%, respectively. Moreover, the effect of improving the oil recovery is closely related to the injection scheme [33]. Zhang et al. used UC11AMPM, SDS, and their mixture as foaming agents, respectively, to prepare CO2 foams, and tested the effect of temperature on the stability of these three foams. With the increase in temperature, the stability of the foams decreased, and the foam produced by the UC11AMPM and SDS mixture had the best temperature resistance [34]. Combining multiple surfactants can achieve better oil recovery effects, but the proportion of different surfactants will have a significant impact on the oil displacement effect. Attarhamed and zoveidavianpoor found that the foaming performance of the mixture of AOS and TX-100 in aqueous CO2 foam was improved compared with that of AOS and TX-100 alone [35]. Memon et al. used AOS, TX-100, and a third surfactant, rose amidopropylamine oxide (LMDO), to control the fluidity of CO2 and improve oil recovery. After the water flooding of Berea sandstone using different combinations of CO2 and surfactant solutions at 1400 psi and 96 °C, surfactant alternating gas (SAG) injection was performed. According to core oil displacement experiments, CO2-SAG based on (0.6 wt% AOS + 0.6 wt% TX-100) achieved the highest recovery rate [36].
There are three main options for introducing surfactants into oil recovery processes. First, CO2 foam is generated from the outside and then injected into the porous medium. Secondly, the surfactant solution and CO2 can be injected together at the same time to form foam in porous media. Thirdly, carbon dioxide and surfactant solutions can be alternately injected, known as SAG injection. The advantages of surfactants mainly lie in reducing viscosity fingering, gravity segregation, and early CO2 breakthrough by changing the magnitude of viscosity and gravity. In addition to fixing CO2, surfactants also tend to reduce the IFT between reservoir fluids, reduce capillary forces, and thus improve crude oil recovery. The synergistic effect of multiple surfactants may produce a better profile control effect than a single surfactant, and this profile control effect is closely related to the proportion of different types of surfactants, which will also an important development direction of foam profile control and flooding in the future.
5. Nanoparticle Injection
An NP (nanoparticle) is defined as a material composed of particles with sizes between 1 nm and 100 nm [37][38]. In terms of CO2-EOR, nanoparticles enhance oil recovery through two pathways: improving the mobility ratio of CO2 to crude oil and reducing asphaltene precipitation during CO2 flooding [39][40]. Lu et al. designed a CO2 core displacement experiment and injected Al2O3 nanoparticles into the core. They found that they adsorbed asphaltene in a solution prepared from toluene and dissolved asphaltene, which means that these NPs can be used to suppress the deposition of asphaltene during CO2 injection in porous media. A concentration of 0.5 wt% nanoparticles and a volume ratio of 0.1 nanofluid slugs to CO2 slugs are considered the best conditions for inhibiting asphaltene damage during CO2 flooding. Compared to the cyclic injection mode, continuous CO2 and nanofluid injection may be more effective. The higher the mass fraction of Al2O3 nanoparticles, the lower the strength of asphaltene precipitation and the greater the decrease in interfacial tension [41]. Other studies have also reached the same conclusion that nanoparticles can reduce the interfacial tension between crude oil and CO2 and reduce asphaltene precipitation in crude oil [42][43]. Ehsan et al. simulated the viscosity increasing effect of Al2O3 nanoparticles with particle diameters of 1 nm, 2 nm, and 3 nm on scCO2 in an environment of 380 K and 20 MPa. Particles with a diameter of 1 nm have the weakest effect on CO2 viscosity, resulting in a 3.67-fold increase in CO2 viscosity. The author also compared the viscosity increasing effect of spherical Al2O3 nanoparticles and columnar CuO nanoparticles on scCO2, and found that the viscosity increasing effect of CuO was 3.4 times lower than that of Al2O3 [44].
Because the surfactant is easy to be adsorbed on the rock surface and decomposes itself, the stability of foam in the formation is poor, and it is not suitable for large-scale application. Nanoparticles can effectively improve the stability of foam in the formation, which has attracted the attention of researchers. At present, there are several views on the mechanism of nanoparticles improving the strength of foam (see Figure 2): (1) nanoparticles will gather at the node intersection of the foam liquid film, hinder the liquid flow between liquid films, reduce the water loss rate of the foam liquid film, and thus improve the stability of the foam liquid film; (2) nanoparticles will form a single layer, double layer, and network of bridging particles between foam liquid films to hinder the coalescence and water loss of the foam, thus improving the stability of the foam [45][46]. Among them, the network aggregation of nanoparticles has the strongest stabilizing effect on foam. AttarHamed et al. investigated the effect of the diameter and concentration of SiO2 nanoparticles on the anionic surfactant effect of α-AOS-CO2 foam stability. The concentrations of the nanoparticles were 0.1 wt%, 0.3 wt%, 0.5 wt%, and 1 wt%, respectively. The diameters of the nanoparticles were 15 nm, 70 nm, and 250 nm. The final experimental results are shown in the figure. When the particle concentration was low, the larger the particle diameter, the better the stability effect of the foam [47]. Bayat et al. compared the stabilization effect of TiO2, CuO, Al2O3, and SiO2 nanoparticles on CO2 foam. When the concentration of nanoparticles was 0.008 wt%, the stabilization effect was the best. When using SiO2 nanoparticles under the same conditions, the maximum increase in crude oil recovery was 17.4%. The main reason for the poor stability of nanoparticles in foam is that nanoparticles are easily adsorbed on the rock surface and agglomerated. Therefore, the better the dispersity of particles in the system, the better the stability of the foam [48][49][50].
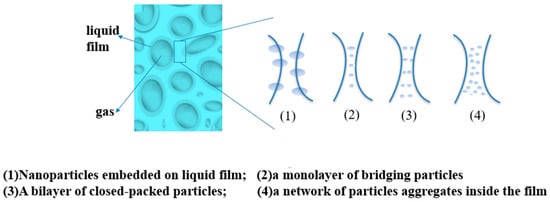
Figure 2. Mechanism of nanoparticles enhancing the stability of foam.
The above four methods for preventing and controlling oil and gas migration in CO2 flooding have their own advantages and disadvantages, as summarized in Table 1. In order to further solve the problem of gas migration during CO2 flooding, in addition to making up for the shortcomings of existing technologies, efforts should also be made to develop new CO2-enhanced oil recovery technologies.
Table 1. Comparison between the different methods of CO2 mobility control.
| Method | Advantage | Disadvantage | Influence Factor |
|---|---|---|---|
| WAG | Reduces CO2 loss and generate economic benefits; Increases the sweep coefficient; Relieves sticky fingering; Delays CO2 breakthrough; Reduces the mobility ratio; Maintains CO2 mixing with crude oil. |
Corrodes pipelines; Unable to alleviate the phenomenon of gravitational differentiation; Initiates asphaltene precipitation; Initiates inorganic salt precipitation; Causes stress damage to the tubing; Causes water lock effect; There are many parameters involved. |
Reservoir factors (temperature, pressure, thickness, porosity, permeability, saturation, heterogeneity); CO2 rheological properties and density; Composition and viscosity of crude oil; Water composition and mineralization; Injection parameters (injection rate, injection pressure, slug ratio); Injection scheme; Spacing between injection and production wells. |
| Polymers for direct thickening of CO2 |
It can be mixed with CO2 to form a thermodynamically stable solution; Increases CO2 density and viscosity; Relieves early CO2 breakthrough and viscous fingering. |
The solubility of polymers is limited by pressure, molecular weight, and molecular chain structure, making it difficult to meet the requirements in many cases. | Reservoir factors (temperature, pressure, thickness, porosity, permeability, saturation, heterogeneity); CO2 rheological properties and density; Composition and viscosity of crude oil; Polymer type, molecular weight, and molecular chain structure; Injection scheme. |
| In situ polymer gels |
Good injectability; Reduces formation heterogeneity. |
Sensitive to reservoir conditions. | Reservoir factors (temperature, pressure, thickness, porosity, permeability, saturation, heterogeneity); CO2 rheological properties and density; Composition and viscosity of crude oil; Injection scheme; Injection parameters (injection pressure, injection rate, injection fluid concentration). |
| Preformed polymer gels |
Reduces formation heterogeneity; Low sensitivity to reservoir. |
Difficulty in injection and inability to act on deep formations; Only applicable to formations with strong heterogeneity or developed fractures. |
Reservoir factors (temperature, pressure, thickness, porosity, permeability, saturation, heterogeneity); CO2 rheological properties and density; Composition and viscosity of crude oil; Injection scheme; Injection parameters (injection pressure, injection rate, injection fluid concentration). |
| Foam | Relieves sticky fingering; Relieves gravity differentiation; Relieves early breakthroughs; Reduces interfacial tension; Changes wettability; Easy to inject; Prevents and controls sedimentation. |
Sensitive to temperature and pressure, prone to cracking; Material exchange with crude oil results in a decrease in stability; Adsorbs on the surface of rocks, resulting in ineffective sealing; Short life cycle; Changes the properties of crude oil. |
Reservoir factors (temperature, pressure, thickness, porosity, permeability, saturation, heterogeneity); CO2 rheological properties and density; Composition and viscosity of crude oil; Injection scheme; Type and concentration of surfactants, molecular structure; Injection parameters (injection rate, injection pressure). |
| Nanoparticle | Reduces the mobility ratio; Prevents and controls asphaltene precipitation; Changes the wettability of rocks; Improves the stability and viscosity of foam; Reduces interfacial tension; Improves CO2 rheological properties. |
Nanoparticles are prone to coalescence, blocking the roar channel, and failing; Large particle sizes can pollute the environment. |
Reservoir factors (temperature, pressure, thickness, porosity, permeability, saturation, heterogeneity); CO2 rheological properties and density; Composition and viscosity of crude oil; Injection scheme; Nanoparticle type, particle size, hydrophilicity, concentration. |
This entry is adapted from the peer-reviewed paper 10.3390/gels9120936
References
- Farajzadeh, R.; Andrianov, A.; Spe, R.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183–184, 1–13.
- Gao, S.; Hu, Z.; Hou, J. Experimental Study on Anti-channeling during CO Flooding for Low Permeability Reservoirs. Spec. Oil Gas Reserv. 2013, 20, 105–108+147.
- Li, X.; Hou, Y.; Gu, Y.; Zhang, C.; Mou, C.; Qi, N.; Pan, L. Experimental study on conductivity of acid etched fractures in dolomite reservoirs. Editor. Dep. Pet. Geol. Recovery Effic. 2021, 28, 88–94.
- Liu, Y.; Liu, Q. A review of channeling blocking gel systems for CO2 flooding. Pet. Geol. Recovery Effic. 2022, 30, 122–134.
- Wang, H. Study on the Gas Channeling Mechanism and Sealing Technology of CO2 Flooding. Master’s Thesis, Northeast Petroleum University, Daqing, China, 2017.
- Bai, B.; Sun, X. Development of Swelling-Rate Controllable Particle Gels to Control the Conformance of CO2 Flooding. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020.
- Panjalizadeh, H.; Alizadeh, A.; Ghazanfari, M.; Alizadeh, N. Optimization of the WAG Injection Process. Pet. Sci. Technol. 2015, 33, 294–301.
- Gbadamosi, A.O.; Kiwalabye, J.; Junin, R.; Augustine, A. A review of gas enhanced oil recovery schemes used in the North Sea. J. Pet. Explor. Prod. Technol. 2018, 8, 1373–1387.
- Awan, A.R.; Teigland, R.; Kleppe, J. A survey of North Sea enhanced-oil-recovery projects initiated during the years 1975 to 2005. SPE Reserv. Eval. Eng. 2008, 11, 497–512.
- Leeuwenburgh, O.; Meekes, S.; Chitu, A. Optimizing CO2—EOR operations by improved conditioning of reservoir models to time-Lapse seismic data. In Proceedings of the Offshore Technology Conference, Rio de Janeiro, Brazil, 29 October 2015.
- Kamali, F.; Hussain, F.; Cinar, Y. An experimental and numerical analysis of water-alternating-gas and simultane-ous-water-and-gas displacements for carbon dioxide enhanced oil recovery and storage. SPE J. 2017, 22, 521–538.
- Han, L.; Gu, Y. Optimization of miscible CO2 water-alternating-gas injection in the bakken formation. Energy Fuels 2014, 28, 6811–6819.
- Mansoori, G.A. Modeling of asphaltene and other heavy organic depositions. J. Pet. Sci. Eng. 1997, 17, 101–111.
- Khormali, A. Effect of water cut on the performance of an asphaltene inhibitor package: Experimental and modeling analysis. Pet. Sci. Technol. 2022, 40, 2890–2906.
- Wang, X.; Gu, Y. Oil recovery and permeability reduction of a tight sandstone reservoir in immiscible and miscible CO2 flooding processes. Ind. Eng. Chem. Res. 2011, 50, 2388–2399.
- Lei, H.; Yang, S.; Zu, L.; Wang, Z.; Li, Y. Oil Recovery Performance and CO2 Storage Potential of CO2 Water-Alternating-Gas Injection after Continuous CO2 Injection in a Multilayer Formation. Energy Fuels 2016, 30, 8922–8931.
- Wang, Q.; Yang, S.; Lorinczi, P.; Glover, P. Experimental investigation of oil recovery performance and permeability damage in multilayer reservoirs after CO2 and water–alternating-CO2 (CO2–WAG) flooding at miscible pressures. Energy Fuels 2019, 34, 624–636.
- Zhang, S.; She, Y.; Gu, Y. Evaluation of polymers as direct thickeners for CO2 enhanced oil recovery. J. Chem. Eng. Data 2011, 56, 1069–1079.
- Zhang, Y.; Huang, S.; Luo, P. Coupling immiscible CO2 technology and polymer injection to maximize EOR performance for heavy oils. J. Can. Pet. Technol. 2010, 49, 27–33.
- Lee, J.J.; Cummings, S.; Dhuwe, A.; Enick, R.; Beckman, E.J.; Perry, R.M.; Doherty, M.; O’Brien, M. Development of small molecule CO2 thickeners for EOR and fracturing. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014; OnePetro: Richardson, TX, USA, 2014.
- O’Brien, M.J.; Perry, R.J.; Doherty, M.D.; Jason, J.L.; Aman, D.; Beckman, E.J.; Robert, M.E. Anthraquinone siloxanes as thickening agents for supercritical CO2. Energy Fuels 2016, 30, 5990–5998.
- Lee, J.J.; Cummings, S.D.; Beckman, E.J.; Robert, M.E.; Ward, A.B.; Mark, D.D.; Michael, J.O.; Robert, J.P. The solubility of low molecular weight Poly (Dimethyl siloxane) in dense CO2 and its use as a CO2-philic segment. J. Supercrit. Fluids 2017, 119, 17–25.
- Bac, J.H.; Irani, C.A. Laboratory investigation of viscosified CO2 process. SPE Adv. Technol. Ser. 1993, 1, 166–169.
- Sakai, T.; Katashima, T. Relationship between physical properties of tetra-PEG gels and polymer chains. Kobunshi 2014, 63, 860–861.
- Sugimura, A.; Asai, M.; Matsunaga, T.; Akagi, Y.; Sakai, T.; Noguchi, H.; Shibayama, M. Mechanical properties of a polymer network of Tetra-PEG gel. Polym. J. 2013, 45, 300–306.
- Al-Ali, A.H.; Schechter, D.S.; Lane, R.H. Application of polymer gels as conformance control agents for carbon dioxide EOR WAG floods. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001; OnePetro: Richardson, TX, USA, 2013.
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532.
- Durucan, S.; Korre, A.; Shi, J.Q.; Govindan, R.; Mosleh, M.H. The use of polymer-gel solutions for CO2 flow diversion and mobility control within storage sites. Energy Procedia 2016, 86, 450–459.
- Binks, B.P.; Campbell, S.; Mashinchi, S.; Piatko, M.P. Dispersion behavior and aqueous foams in mixtures of a vesicle-forming surfactant and edible nanoparticles. Langmuir 2015, 31, 2967–2978.
- Hosseini-Nasab, S.M.; Zitha, P.L.J. Investigation of certain physicalchemical features of oil recovery by an optimized alkalisur-factantfoam (ASF) system. Colloid. Polym. Sci. 2017, 295, 1873–1886.
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178.
- Yan, W.; Miller, C.A.; Hirasaki, G.J. Foam sweep in fractures for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 348–359.
- Ren, G.; Nguyen, Q.P. Understanding aqueous foam with novel CO2-soluble surfactants for controlling CO2 vertical sweep in sandstone reservoirs. Pet. Sci. 2017, 14, 330–361.
- Zhang, P.; Diao, Y.; Shan, Y.; Pei, S.; Ren, S.; Zhang, L.; Yang, H. Experimental investigation of amine-surfactant CO2 foam for smart mobility control during CO2 flooding. J. Pet. Sci. Eng. 2020, 184, 106511.
- AttarHamed, F.; Zoveidavianpoor, M. The foaming behavior and synergistic effect in aqueous CO2 foam by in situ physisorption of alpha olefin sulfonate and Triton X-100 surfactants and their mixture. Pet. Sci. Technol. 2014, 32, 2376–2386.
- Memon, M.K.; Elraies, K.A.; Al-Mossawy, M.I. Impact of new foam surfactant blend with water alternating gas injection on residual oil recovery. J. Pet. Explor. Prod. Technol. 2017, 7, 843–851.
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, J.P.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- Jafari, S.; Khezrnejad, A.; Shahrokhi, O.; Ghazanfari, M.H.; Vossoughi, M. Experimental investigation of heavy oil recovery by continuous/WAG injection of CO2 saturated with silica nanoparticles. Int. J. Oil Gas Coal Technol. 2015, 9, 169–179.
- Kazemzadeh, Y.; Malayeri, M.R.; Riazi, M.; Parsaei, R. Impact of Fe3O4 nanoparticles on asphaltene precipitation during CO2 injection. J. Nat. Gas Sci. Eng. 2015, 22, 227–234.
- Lu, T.; Li, Z.; Fan, W.; Zhang, X.; Lv, Q. Nanoparticles for inhibition of asphaltenes deposition during CO2 flooding. Ind. Eng. Chem. Res. 2016, 55, 6723–6733.
- Hassanpour, S.; Malayeri, M.R.; Riazi, M. Utilization of Co3O4 nanoparticles for reducing precipitation of asphaltene during CO2 injection. J. Nat. Gas Sci. Eng. 2016, 31, 39–47.
- Hassanpour, S.; Malayeri, M.R.; Riazi, M. Asphaltene Precipitation during Injection of CO2 Gas into a Synthetic Oil in the Presence of Fe3O4 and TiO2 Nanoparticles. J. Chem. Eng. Data 2018, 63, 1266–1274.
- Mahdavi, E.; Khaledialidusti, R.; Barnoush, A. Rheological properties of super critical CO2 with Al2O3: Material type, size and temperature effect. J. Mol. Liq. 2019, 289, 111037.
- Fehr, A.; Telmadarreie, A.; Berton, P.; Bryant, S. Synergy Between Commodity Molecules and Nanoparticles as Steam Mobility Control Additives for Thermal Oil Recovery. In SPE Annual Technical Conference and Exhibition? SPE: Houston, TX, USA, 2020.
- Rognmo, A.U.; Al-Khayyat, N.; Heldal, S.; Vikingstad, I.; Eide, O.; Fredriksen, S.B.; Alcorn, Z.P.; Graue, A.; Bryant, S.L.; Kovscek, A.R.; et al. Performance of silica nanoparticles in CO2 foam for EOR and CCUS at tough reservoir conditions. SPE J. 2020, 25, 406–415.
- Attarhamed, F.; Zoveidavianpoor, M.; Jalilavi, M. The incorporation of silica nanoparticle and alpha olefin sulphonate in aqueous CO2 foam: Investigation of foaming behavior and synergistic effect. Pet. Sci. Technol. 2014, 32, 2549–2558.
- Bayat, A.E.; Rajaei, K.; Junin, R. Assessing the effects of nanoparticle type and concentration on the stability of CO2 foams and the performance in enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 222–231.
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid. Interface Sci. 2008, 13, 134–140.
- Daryasafar, A.; Shahbazi, K. Using nanotechnology for CO2-foams stabilization for application in enhanced oil recovery. Int. J. Energy A Clean Environ. 2018, 19, 217–235.
This entry is offline, you can click here to edit this entry!
