1. Pituitary Adenylate Cyclase-Activating Peptide and Vasoactive Intestinal Peptide
PACAP is a multi-functional peptide that has therapeutic potential in a variety of pathophysiological conditions and represents a promising avenue for intervention. PACAP is a neuropeptide that plays a crucial role in both neural and endocrine functions [
78]. This peptide is widely distributed throughout the body and is involved in diverse physiological processes, including circadian rhythm and immune system regulations, modulation of pain perception, and stress response [
79]. PACAP also has neuroprotective effects and has been shown to support nerve cell survival and regeneration in various neurological disorders [
80]. GPCRs control the signaling pathways and cause the activation of adenylate cyclase (AC), the release of cyclic AMP, and the activation of protein kinase A (PKA) and calcium channels [
81,
82]. PACAP is a multi-functional peptide that has therapeutic potential in a variety of pathophysiological conditions and represents a promising avenue for therapeutic intervention [
83].
2. Background
PACAP was found in ovine hypothalamic extracts in 1989. It is a 38-amino acid peptide hormone that stimulates AC activity in the pituitary gland [
84]. Subsequently, it was found to be widely distributed in the central and peripheral nervous systems, as well as in non-neural tissues, including the adrenal gland, pancreas, gut, and reproductive system [
85]. PACAP exists in three biologically active forms: PACAP1–38, 6–38-amino acid form of PACAP (PACAP6–38), and PACAP1–27 [
86]. PACAP-related peptide (PRP) is also a member of the PACAP family [
87]. Radioimmunoassay demonstrated that PACAP1–38 levels were approximately 60 times greater than PACAP1–27 levels and 10 times greater than PRP levels [
88].
Since its discovery, PACAP has been extensively studied for its potent neuroprotective effects against a diverse range of neurological disorders, including stroke, traumatic brain injury, Parkinson’s disease, and Alzheimer’s disease [
89,
90]. Recent findings suggest that PACAP may also play a key role in the regulation of immune cell function and cytokine production, highlighting its potential as a therapeutic target for immune-mediated diseases such as rheumatoid arthritis, multiple sclerosis, and asthma [
91]. Furthermore, PACAP has been implicated in the regulation of energy metabolism, making it a promising therapeutic agent for the treatment of metabolic disorders such as obesity and diabetes [
92]. Overall, the growing body of evidence on the multifunctional properties of PACAP highlights its potential as a novel therapeutic target for a wide range of diseases.
VIP is a 28-amino acid polypeptide that was first characterized in 1970. It is secreted by cells throughout the intestinal tract and is widespread in many internal organs and systems [
93]. VIP plays important roles in many biological functions, such as stimulation of contractility in the heart, vasodilation, promoting neuroendocrine–immune communication, lowering arterial blood pressure, and anti-inflammatory and immune-modulatory activity [
94]. VIP stimulates the secretion of electrolytes and water by the intestinal mucosa and acts as a neurotransmitter, inducing a relaxation effect in some tissues [
95]. VIP is also involved in the pathophysiology of various diseases, including osteoarthritis, cancer, and autoimmune disorders [
94]. Furthermore, VIP is implicated in the physiological and pathophysiological roles of migraine [
96].
3. Receptor and Signaling Mechanisms of PACAP and VIP
PACAP plays an important role in a wide range of biological processes such as feeding behavior, stress response, neuroprotection, and regulation of neurotransmitter release. It activates three different GPCRs named PAC1, vasoactive intestinal peptide receptor (VPAC) 1, and VPAC2; these receptors are widely expressed in the central and peripheral nervous systems, endocrine systems, and immune systems [
97]. The binding of PACAP to these receptors leads to the activation of multiple signaling mechanisms [
98].
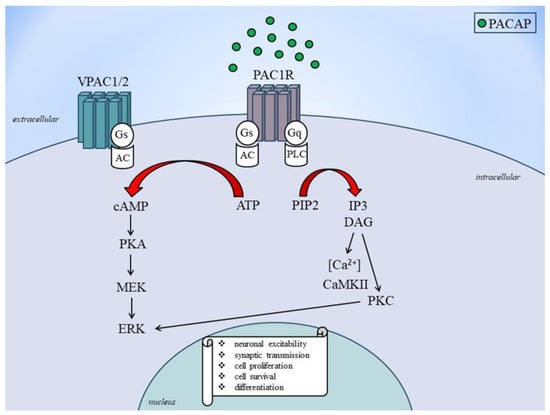
Activation of the PAC1 receptor by PACAP leads to the activation of the adenylyl cyclase enzyme, which in turn leads to the production of cyclic adenosine monophosphate (cAMP) and the activation of PKA [
99]. It also triggers the activation of phospholipase C, which leads to the breakdown of phosphatidyl inositol 4,5-bisphosphate (PIP2) into inositol triphosphate and diacylglycerol (DAG), which activates protein kinase C (PKC) [
100]. On the other hand, VPAC1 and VPAC2 receptor activation leads to AC enzyme activation, which leads to the generation of cAMP and the activation of PKA [
101]. Also, PACAP signaling turns on calcium signaling, which causes intracellular calcium to be released and calcium/calmodulin-dependent kinase II to be activated [
102]. PACAP signaling also activates the mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and jun N-terminal kinase signaling pathways [
103]. These signaling mechanisms contribute to the diverse biological effects of PACAP on cellular functions. The regulation of PACAP gene expression is presented in
Figure 2.
Figure 2. PACAP receptors signaling to ERK activation. AC, adenylate cylase; ATP: adenosine monophosphate; cAMP: cyclic adenosine monophosphate; DAG: diacylglycerol; ERK, extracellular signal-regulated kinase; Gs and Gq: stimulatory G protein; MEK: mitogen-activated protein kinase kinase; PKA: protein kinase A; PKC: protein kinase C; PACAP: pituitary adenylate cyclase-activating polypeptide; PAC1: PACAP 1 receptor; PIP2: phosphatidylinositol bisphosphate; VPAC1: vasoactive intestinal peptide receptor type 1; VPAC2: vasoactive intestinal peptide receptor type 2.
PACAP and VIP are neuropeptides that interact specifically with three receptors (VPAC1, VPAC2, and PAC1) from the class II B GPCR family [
104]. The similarities between PACAP and VIP in receptor and signaling mechanisms include the following: PACAP and VIP share nearly 70% amino acid sequence identity; PACAP binds with high affinity to all three receptors, while VIP binds with high affinity to VPAC1 and VPAC2 receptors and has a thousand fold lower affinity for the PAC1 receptor compared to PACAP; both PACAP and VIP receptors are preferentially coupled to Gαs, leading to activation of AC, subsequent cAMP production, and activation of PKA; and PKA may in turn activate ERKs, PACAP and VIP receptor-mediated signaling pathways [
105,
106,
107,
108]. Due to the wide distribution of VIP and PACAP receptors in the body, potential therapeutic applications of drugs targeting these receptors, as well as expected unwanted side effects, are numerous [
109]. Designing selective therapeutics targeting these receptors remains challenging due to their structural similarities.
4. Role of PACAP and VIP in Migraine
PACAP has been strongly associated with the pathophysiology of migraine. PACAP is found in high levels in the trigeminal nerve, which is known to play a critical role in this condition. PACAP is known to increase the sensitivity of the trigeminal nerve, cause dilation of blood vessels in the brain, and trigger inflammation. All these biological effects have been implicated in the development of migraine attacks [
110]. Several studies have been conducted to investigate the role of PACAP in migraine. One study showed that PACAP levels in the blood are significantly higher in migraine patients during an attack compared to headache-free controls [
111]. This study suggests that PACAP could be used as a potential biomarker for migraine. Another study demonstrated that the venous infusion of PACAP into migraine patients resulted in the development of migraine-like attacks [
112]. This finding strongly supports the hypothesis that PACAP plays a crucial role in the pathophysiology of migraine and suggests that blocking PACAP could be a potential therapeutic target for the treatment of migraines. The role of PACAP in migraine pathology is well established, and there is strong evidence that this neuropeptide plays a crucial role in the development of migraine attacks. Further research is needed to better understand the mechanism of action of PACAP and to develop new pharmacological agents that target PACAP for the treatment of migraines.
Both CGRP and PACAP are multifunctional peptides with many roles in the nervous, cardiovascular, respiratory, gastrointestinal, and reproductive systems. They play a role in vasodilation, neurogenic inflammation, and nociception. While CGRP plays an integral role in migraine, PACAP is likely to play a similar but distinct role as CGRP based on similarities and differences observed in both clinical and preclinical studies [
113]. In rodent models, the PACAP pathway appears to be independent of the CGRP pathway, suggesting that CGRP and PACAP act in parallel ways that cause a migraine-like symptom [
114]. In migraine without aura, the first double-blinded placebo-controlled study reported that 33% of the patients developed delayed migraine attacks after CGRP administration [
115]. The studies have identified the involvement of two endogenous neuropeptides, CGRP and PACAP, in the pathogenesis of migraines [
116].
VIP has also been implicated in the pathophysiology of migraine [
117]. The similarities between PACAP and VIP in their roles in pathogenesis include the following: PACAP and VIP are released in conjunction with migraine and cluster headache attacks [
118]; PACAP and VIP are potent vasodilators and can cause migraine-like attacks when infused into people [
119]; a 2-h infusion of VIP caused migraine attacks, indicating that VIP plays a significant role in pathophysiology and intravenous administration of PACAP-38 caused headaches in all healthy subjects and migraine-like attacks in 58% of patients with a history of migraine without aura [
15,
35]; PACAP and VIP receptors are preferentially coupled to Gαs, leading to activation of AC, subsequent cAMP production, and activation of PKA [
120]; PKA may in turn activate ERKs [
121]; PACAP and VIP receptor-mediated signaling pathways are shown to share activities, including vasodilation, neurogenic inflammation, and nociception in rodents [
122]; PACAP and VIP receptors provide a rich set of targets to complement and augment the current CGRP-based migraine therapeutics; VPAC1 receptors play a dominant role in PACAP-induced vasorelaxation in female mice [
123]. Also, PG 99-465, a selective VPAC2 receptor antagonist that has been used in a number of physiological studies, has been shown to have significant activity at VPAC1 and PAC1 receptors [
124].
5. Preclinical Studies
In addition to in vitro systems, a variety of organisms are used in experimental medicine [
125,
126,
127]. Understanding the effects of endogenous neuropeptides, neurohormones, and metabolites has advanced significantly thanks to the information gathered using laboratory animals [
128,
129,
130,
131,
132,
133]. Animal models are a crucial tool for bridging the knowledge gap between data- and hypothesis-driven benchwork and its application to clinical bedside management. PACAP has been extensively studied as a neuromodulator in the trigeminal nociceptive pathway [
134]. Preclinical studies have shown that PACAP is involved in the transmission of pain signals from the periphery to the central nervous system and is therefore a potential target for the treatment of migraine and other headache disorders [
135,
136].
In animal models, PACAP has been shown to play a role in trigeminal sensitization, which is the process by which nociceptive signals become amplified and persistent, leading to chronic pain [
137]. Studies have also found that PACAP is involved in the activation of inflammatory pathways in the trigeminal nerve, further contributing to pain and inflammation [
138]. In addition, PACAP has been implicated in the regulation of blood flow to the brain, which may also play a role in headache pathophysiology [
139] and other neurological [
26] or neuropsychological conditions [
88]. In an experimental model of migraine, intraperitoneal administration of nitroglycerol caused marked photophobia and meningeal vasodilatation, and increased the number of c-fos-positive activated neurons in the TNC in wild-type mice but not in PACAP1–38-deficient mice [
140]. In line with this, an increased concentration of PACAP1–38 was detected in the TNC after the activation of the TS in different animal models [
141,
142].
PAC1 receptor antagonists include PACAP6–38, N-stearyl-[Nle17] neurotensin-(6-11)/VIP-(7-28), deletion mutants of maxadilan, M65, and Max.d.4, and synthesized small-molecule acyl hydrazides, including PG 97-269 [
143]. PACAP6–38 has been used as a PAC1 receptor antagonist in many studies, but it has an affinity for VPAC2 receptors [
144]. N-stearyl-[Nle17] neurotensin-(6-11)/VIP-(7-28) (SNV) is a chimeric peptide analog that antagonizes the VIP2/PACAP receptor subclass. SNV is a better mitogen for the keratinocytic cell line and can increase AC activity in rat brain membranes 100 times more than VIP1-28 [
145,
146]. No migraine-related studies have been documented. The maxadilan is a vasodilator peptide derived from the salivary glands of sandflies. Its deletion mutants, M65 and Max.d.4, have been reported to be selective PAC1 receptor antagonists but have not been extensively used due to problems of availability [
147,
148]. PG 97-269 is a selective VPAC1 receptor antagonist with negligible affinity for the PACAP1 receptor. It did not stimulate AC activity but inhibited competitively the effect of VIP on AC activity in cells expressing the VIP1 receptor [
146]. VIP and PACAP-induced vasodilation were partially blocked by PG 97-269, indicating that PACAP and VIP may play a role in migraine pathophysiology and that PG 97-269 may have therapeutic potential for migraine [
149]. Thus, preclinical studies suggest that concentrating on the PACAP signaling pathways in the trigeminal nociceptive system could be an effective strategy for discovering novel treatments for headache disorders. However, more research is needed to fully understand the mechanisms underlying PACAPs’ role in headache pathophysiology and to develop effective and safe PACAP-targeted therapies.
VIP plays a key role in sensory processing and the modulation of pain pathways in the trigeminal system. In preclinical studies, VIP has been shown to change the activity of nociceptive neurons in the trigeminal ganglion and make the TNC more sensitive, which can cause chronic pain or migraines [
150]. In response to noxious stimuli, the trigeminal sensory neurons release VIP. This can activate VIP receptors on nearby neurons and cause the release of a number of signaling molecules involved in pain amplification [
151]. VIP-mediated sensitization of trigeminal neurons can lead to hyperexcitability and increased responsiveness to noxious stimuli, which may contribute to the development and maintenance of chronic pain or migraine [
152]. Targeting VIP signaling pathways may therefore represent a promising approach for the development of novel therapies for chronic pain or migraine.
6. Clinical Studies
A growing body of clinical research suggests that PACAP plays an important role in migraine pathophysiology. Patients with migraines exhibit higher levels of PACAP compared to control groups [
153]. PACAP is a neuropeptide recognized for its involvement in the activation of nociceptive pathways, contributing to the development of migraines. The high levels of PACAP in migraineurs have been associated with increased headache severity and frequency, and this has led to the exploration of PACAP as a therapeutic target for treatment [
154]. In migraineurs without aura, the development of PACAP1–38-evoked migraine-like attacks was independent of the severity of family load [
35,
155]. In the same study, 90 min after the injection, the levels of numerous migraine-related molecular markers were increased in the plasma of patients [
156]. Magnetic resonance imaging angiography examinations revealed that PACAP1–38-induced headache was associated with prolonged vasodilatation of the middle meningeal artery (MMA) but not the middle cerebral artery (MCA). Sumatriptan, an antimigraine medication, was able to alleviate the headache, which mirrored the contraction of the MMA but not the MCA, indicating that PACAP1–38-induced headaches may originate from extracerebral arteries [
157].
An increasing number of clinical studies have shown that targeting PACAP signaling may be a promising therapeutic strategy for migraine treatment. In terms of safety, PACAP has been generally well tolerated in clinical trials [
158]. One study found that PACAP induces headaches via sustained vasodilation and that targeting the PACAP pathway may be a promising approach for treatment [
159]. AMG 301, a mAb that targets the PAC1 receptor, was administered to patients with episodic or chronic migraines in a randomized, double-blind, placebo-controlled phase 2 study. There was no significant difference between the AMG 301 group and the placebo group, suggesting that AMG 301 was ineffective for prevention [
160,
161]. On the other hand, the PACAP ligand mAb, Lu AG09222, was shown to reduce the number of monthly migraine days from baseline to weeks 1–4 of treatment statistically significantly more than placebo [
162,
163]. Additionally, the mAb targeting the PAC1 receptor, LY3451838, is currently undergoing phase 2 clinical trials for adults with treatment-resistant migraine. This trial is in progress, and the results are not yet available [
164]. Overall, the efficacy and safety of PACAP as a migraine treatment in clinical studies suggest that it is a promising option for patients with this debilitating condition. Further research is needed to fully understand the potential of PACAP as a treatment for migraines, but the current evidence is encouraging.
VIP infusion has been studied in the context of migraines, with a particular focus on its potential to provoke migraine attacks and its role in pathophysiology. A phase 2 clinical trial investigated the effects of a long-lasting infusion of VIP on headaches, cranial hemodynamics, and autonomic symptoms in episodic migraine patients without aura [
165]. The study found that a 2-h infusion of VIP promoted long-lasting cranial vasodilation and delayed headaches in healthy volunteers, resembling the effect of prophylaxis. However, other studies have suggested that VIP infusions may actually provoke migraine attacks. For example, a randomized clinical trial found that a 2-h infusion of VIP caused migraine episodes, suggesting an important role of VIP in migraine pathophysiology [
15]. It remains unclear whether the lack of migraine induction can be attributed to the only transient vasodilatory response after a 20-min infusion of VIP. Overall, the search results suggest that VIP infusion may have a role in migraine pathophysiology, but further research is needed to fully understand its effects and potential therapeutic applications.
This entry is adapted from the peer-reviewed paper 10.3390/cells12222649