Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Neurons and glia (astrocytes, radial glia, oligodendroglia, and microglia) are the neural cells of the central nervous system (CNS). Glial cells have different functions; microglia are the resident macrophages in the CNS, oligodendrocytes are responsible for myelin production, NG2-positive glia is consistent with an oligodendrocyte progenitor function, and astrocytes play an important role both in homeostasis and in diseases.
- astrocytes
- inflammation
- learning
- memory
- neurons
- neurodegeneration
- neurodevelopment diseases
- oxidative stress
- sleep disorders
1. Astrocyte Properties and Functions
Astrocytes contact different populations around neurons, and this is true for protoplasmic astrocytes, which normally contact neurons in the gray matter, and for fibrous astrocytes that contact neuronal extensions [1]. The functions performed by astrocytes are, among others, to control the sleep process, form the extracellular matrix, serve as a support, build and regulate the blood–brain barrier, maintain the balance of extracellular ions, control the production of neurotransmitters, and modulate the synapse (tripartite synapse hypothesis) [2][3]. Memory consolidation occurs during the sleep period and astrocytes can control this process by communicating with other brain cells. During the sleep period they clean toxins, neurotransmitters and release water, ions, and molecules [4].
Researchers believe that neurons and astrocytes are the essential elements in all regulating the processes related to memory of lived situations or dreams. In the CNS, only neurons can produce electrical impulses and therefore help in memory formation and storage. Just like when we turn off a computer and turn it on again after two years, the information stored in it remains, memory created via the communications between neurons remains. We do not know how long-term memory is produced and how memories are maintained but it is established that neurons need to be active all the time to carry out electrical impulses for that memory to be created and stored. Does this imply that we need to have neurons firing constantly if we want to remember something? Astrocytes modulate Ca2+ variations and it is possible that using other pathways and signals they are able to communicate with each other and with neurons over time [5]. In fact, in neurodevelopmental disorders, such as autism [6], different forms of schizophrenia [7], and early onset bipolar disorder [8], the number and morphology of astrocytes changes from one situation to another. In bipolar disorder, patients have an increase in brain functions when they are in the optimistic phase and, when they are in the depressive phase they almost find it impossible to register and develop any work in the brain. The change from an optimistic situation to that of depression is not prompt; it takes days, weeks and/or even months, but it is constant. The functions of the neurons cannot explain the above phenomenon since they are cells that at times are activated or at times not (the cell fires or not), and the modulation of the neuronal action potential is impossible. On the contrary, there is a network between the astrocytes and when necessary, a great communication between them and with the neurons, oligodendroglia and microglia is initiated, which is maintained over time [5]. In the evolution of Homo sapiens, the increase in size and complexity of astrocytes coincided with an increase in intelligence [9]. Furthermore, the difference between humans and rats found is the diameter of the average domain of a protoplasmic astrocyte (2.5 times larger), the volume (bigger in humans) and the arborization. Moreover, fibrous astrocytes are 2.3 times larger in humans than in rats [9][10][11].
Currently, a new field has been opened for the study of the role of astrocytes in the nervous system and their possible role in memory. This could provide a new direction for future interventions in CNS diseases.
2. Glutamine, Glutathione and Astrocytes
Another function of astrocytes is to supply glutamine to neurons and remove glutathione from the synaptic bouton. In AD, neurons die because of the hyperphosphorylation of TAU (protein π) (pTAU) protein. Astrocytes are probably involved in this process because glutamine reduction and/or glutathione removal may be affected in these patients. In addition, a reduction in ATP produced by damaged mitochondria is detected [4].To function adequately, our brain requires oxygen, which flows through the circulatory system to the extracellular space between brain cells. If the heart function is correct, enough oxygen goes into the brain crossing the blood–brain barrier. On the contrary, if enough oxygen does not go into the brain, problems in brain cell functionality will occur with decrement in the production of adenosine triphosphate (ATP). So, cardiology specialists, neurologists, psychologists, and medical specialists must remain vigilant from now on and in the future while diagnosing AD [12]. Moreover, since astrocytes are involved in non-physiological pain [13], discerning the communication between the cells of the sensory ganglia could be important in the treatment of chronic pain [14][15] (Figure 1).
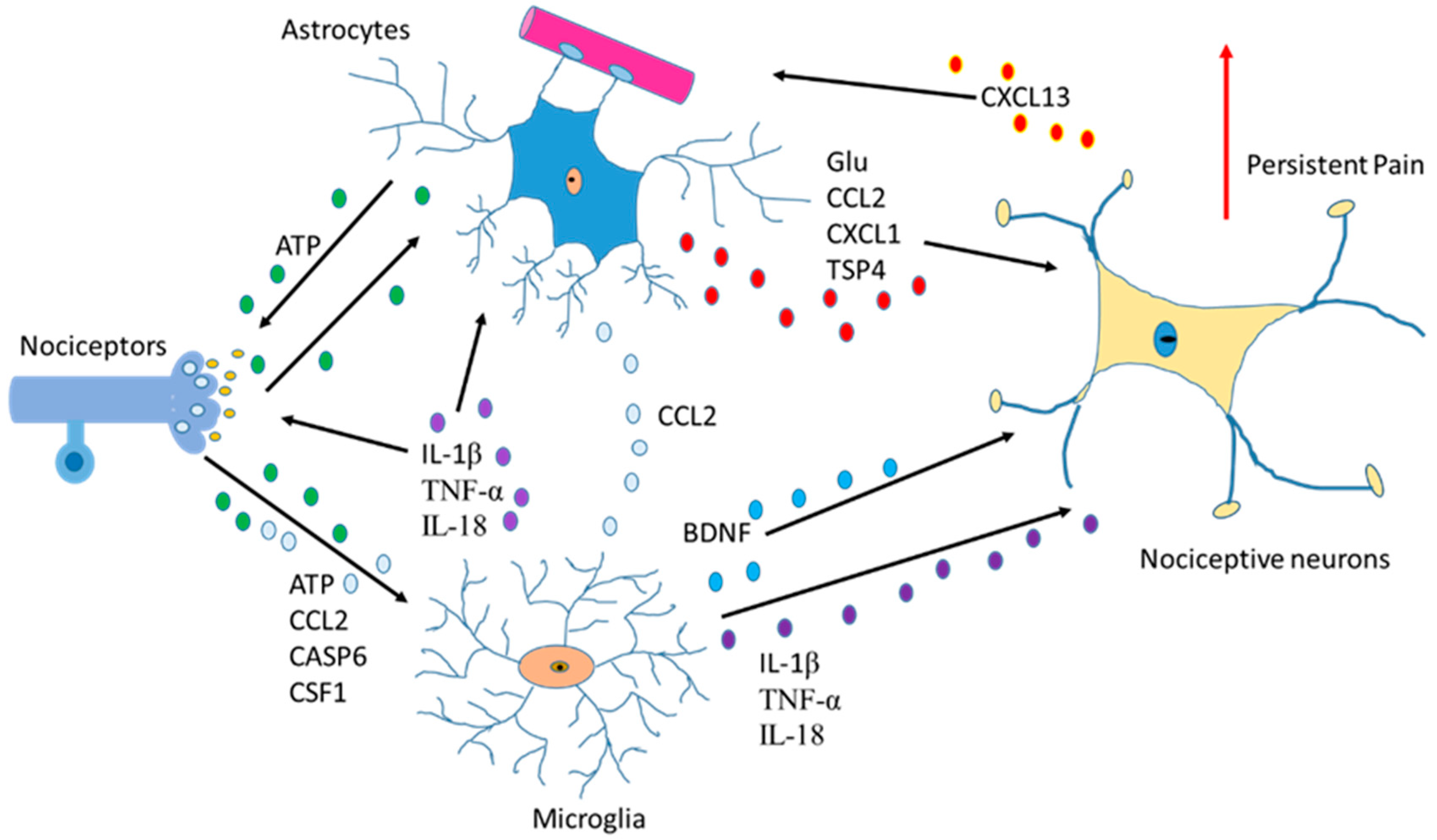
Figure 1. Communication between astrocytes, neurons, and microglia in pain situations. Activation of nociceptors causes the induction of cytokines, chemokines, BDNF and neurotrophic factors producing changes an increase in persistent pain.
3. Neurodegeneration Mechanisms
In the neurodegenerative cascade, several basic mechanisms can join in, such as apoptosis, necrosis, autophagy, retrograde neurodegeneration, Wallerian degeneration, demyelination and astrogliopathy [16]. There is evidence of apoptotic mechanisms in animal models of various neurodegenerative diseases, but the evidence in human tissues is limited. The activation of caspase 1, 3, 8 and 9 and the release of cytochrome c observed in models of Huntington’s disease (HD) is demonstrated in human striated brain tissue [17][18]. Similarly, caspases activation and neuronal apoptosis have been detected in ALS [19] and HIV [20]. In necrosis, with non-caspases-dependent death, two major effector proteins act, serine/threonine-protein kinase 1 (RIPK1) and the mixed lineage kinase domain (MLKL). In murine ALS models, release of TNF-α, FasL and TRAIL by astrocytes has been detected that can trigger necrosis through the activation of RIPK1 and MLKL [20]. Furthermore, in humans with ALS, a normal pathology mediated by RIPK1 has been detected [21]. On the other hand, in MS, necrotic mechanisms are also observed in pathological samples [22].
Apathy has multifactorial symptoms, such as behavioral, cognitive, and emotional facets including impaired motivation and reduced goal-directed behavior. Apathy belongs to schizophrenia, bipolar disorders, and autism’s negative symptomatology, although the molecular mechanisms are still poorly studied [23][24]. Correlations between apathy with specific brain regions and executive functions have been shown (the anterior cingulate cortex, orbitofrontal cortex, and the ventral and dorsal striatum). It is considered the major neuropsychiatric symptom in both acquired and neurodegenerative disorders, such as strokes [25], AD [26], ELA [27] or Parkinson’s disease [28]. All these disorders have a disturbance in the normal balance of neurotransmitters and are associated with anomalies in specific brain regions and inflammatory pathways leading to glia activation and finally neuronal and neural loss [29]. In MS there is a decomposition of the blood–brain barrier (BBB), death without regeneration of oligodendrocytes, loss of myelin, axonal degeneration and reactive gliosis of astrocytes and activation of microglia [30][31]. In this disease, inflammation plays an important role by increasing in cytokines and chemokines. In the pathophysiology of MS, the BBB is compromised, causing the activation of the microglia and the immune cells of the periphery. The microglia not only produces pro-inflammatory cytokines and chemokines secretion with decreased anti-inflammatory agents but also releases reactive oxygen and glutamate species [32]. Each type of cell of the innate and adaptive immune system can organize the inflammatory response within the CNS and the autoreactive CD4 + T cells make an important contribution in the MS.
4. Astrocytes, Sleep Process and Diseases
A century ago, Santiago Ramón y Cajal proposed astrocytes as cells that regulate the process of sleep [4]. He detected several processes in the synapses during sleep and observed retraction of those processes during wakefulness. In 2009, scientists began to take an interest in sleep and the role of astrocytes in it and discovered that the influence of astrocyte on the sleep/wake cycle [33] and then, astrocytes were postulated as modulator members of the homeostasis of the sleep cycle [34]. Astrocytes release adenosine units to its receptor, adenosine A1, occasioning sleep and driving to total sleep [35]. Furthermore, it is known that astrocytes clean the brain during sleep, releasing solutes and water inside the brain, cleaning it through astrocytic aquaporins 4. In addition, in vivo microdialysis studies have shown that β-amyloid (the toxic peptide in Alzheimer’s brain) increases within the interstitial fluid during wakefulness and decreases during the sleep process [35]. Thus, the brain cleaning that occurs during the sleep period is decreased in Alzheimer’s patients compared to control individuals [36][37]. In addition, the changes observed in the amount of toxic peptide decrease during the development of AD [38], so detection in the glymphatic pathway could be affected in neurodegenerative patients and in other diseases, such as bipolar disorders, chronic fatigue syndrome, MS and schizophrenia [39]. Furthermore, the sleep/wake cycle, modulated by astrocytes, is also altered in those diseases [40][41][42]. Many people suffering from these diseases present a REM (Rapid Eye Movement) alteration process during the sleep period. This data could indicate alterations in the sleep period of the active zone of the brain that initiates the off-switch in the brain. In fact, the patients cannot sleep well, and they feel tired during the awake period, with problems in attention, memory, spatial recognition, and so on.
5. Therapeutic Effects to Combat Diseases
At present, there are many therapeutic drugs used against diseases of the nervous system, however, it is also important to test the use of new drugs and approaches against those diseases to treat them more effectively (Table 1). Future therapeutics against brain diseases must develop specific drugs against reactive astrocytes and microglia activation. Studies on the mechanisms that eliminate amyloid beta toxic peptide, the decrement of the pTAU inside the neurons, the ATP changes in the brain controlled by astrocytes and the production of metabolites, will be necessary for finding therapeutic targets in AD and other diseases [43].
Crises due to sensory overstimulation in people with autism are involuntary. The body of a person with autism react to sensory stimuli and the person being unable to bear the overstimulation suffers from a nervous breakdown. Some approaches could produce beneficial physiological effects, such as the reduction in deep pressure to achieve a decrement in anxiety in children with autism spectrum disorders (ASD) [44][45]. Furthermore, the use of new drugs, such as monoclonal antibodies designed to elicit an immune response to eliminate senile plaques, which damage communication between brain cells and end up killing the neurons, will be necessary. In chronic pain, drugs controlling the mechanisms of SG cells and the interaction of these cells with the neuronal body will be important to assume the relationship between astrocytes and the other cells in the sensorial ganglia and chronic pain treatment. In the future, for better health, brain changes in sleep-inducing proteins and the sleep/wake cycle could help fighting sleeping disorders and neurodegenerative diseases as well. In addition, the influence of astrocytes in the brain cleaning process will be a therapeutic approach to eliminate the toxic elements detected in many diseases, such as in AD, Parkinson’s, or ALS (Amyotrophic Lateral Sclerosis) and many other neurodegenerative diseases in which toxic proteins are present. Moreover, altered mRNA expression profile in ALS and/or other neurodegenerative diseases has been detected with an increase in inflammation produced by microglia and astrocyte reactivities, which are potential mediators of neurodegenerative processes [46][47].
Table 1. Drugs and their effects on glia.
| DRUGS | EFFECT ON ASTROCYTES | REFERENCES |
|---|---|---|
| IFN-β | Downregulation of cytokine, NO production, and MMP generation | Bhat et al., 2019 [48] |
| Pioglitazones | Inhibit inflammation reducing glial activation | Dhapola et al., 2021 [49] |
| Fingolimod | Immune-modulator inhibitor actions on activated microglia | Pitteri et al., 2018 [50] |
| Minocycline | Immune-modulator inhibitor actions on activated microglia | Wang et al., 2020 [51] |
| Xaliproden | Production of increased amount of neurotrophic factors | Lacomblez et al., 2004 [52] |
| Cyclooxygenase (COX) inhibitors | Improved memory and decrease amyloid deposition | Lopez-Ramirez et al., 2021 [53] |
| Propentofylline | Decrement of neuroinflammation relative to glial cell activation | Sweitzer and De Leo, 2011 [54] |
| Glatiramer acetate | Anti-inflammatory cytokine and neurotrophic factors increase | Kasindi et al., 2022 [55] |
| Verapamil + magnesium sulphate | Block overactivation of L-Type calcium channels | Zhang et al., 2019 [56] |
| Rapamycin | Astrogliosis inhibition | Selvarani et al., 2021 [57] |
| Fuoxetine | Decrement of antigen-presenting | Barakat et al., 2018 [58] |
| Teriflunomide | NO synthesis downregulation | Hauser et al., 2020 [59] |
| Methotrexate | Induction of astrogliosis, injury | Shao et al., 2019 [60] |
| Tacrolimus | Inhibition of pro-inflammatory cytokines | La Maestra et al., 2018 [61] |
| Mycophenolate mofetil | Downregulation of NO synthesis | Ebrahimi et al., 2012 [62] |
| Glutocorticoids | Dwonregulation of pro-inflammatory cytokines, and astrogliosis | Nichols et al., 2001 [63] |
It is necessary to promote the clearance of toxic proteins by astrocytes (such as amyloid beta) via different mechanisms, such as autophagy or ubiquitin systems. In addition, in reactive astrocytes, an increase in antioxidant proteins, such as Nrf2 (Nuclear Factor Erythroid 2-related factor 2), could benefit our brain. On the other hand, astrocytes can clear toxic peptides from the brain leading to reactive astrocytes and increased inflammation, toxic proteins, and oxidative stress molecules. Regulation of the oxidative stress state and inflammation could help neurons located near astrocytes to survive. Additionally, astrocytes can increase GABA levels after damage, so controlling MAO-B (monoamine oxidase-B) activity could help rescue the brain from memory problems, such as those found in the AD. Cerebral blood flow (CBF) decreases with age. Between the ages of 20 and 60, the CBF falls by 16% and continues to fall by 0.4% each year. A reduction in oxygen and glucose supply to the brain occurs, and this drop in CBF reduces ATP energy production. Mitochondrial loss or damage with reduced ATP worsens when vascular risk factors (VRFs) develop during Alzheimer’s disease and may accelerate CBF declination and mitochondrial deficiency where mild cognitive impairment (MCI) develops [12]. One form of photobiomodulation (PBM), transcranial infrared brain stimulation (TIBS), is planned in a randomized, placebo-controlled study of MCI patients which is to be conducted at our university. Photobiomodulation has been used in Parkinson’s disease, depression, traumatic brain injury and stroke with reported benefits. Medical interventions, pharmacological approach, etc. have been used in AD, but TIBS will be a better technique for the future. The study of the effects of photobiomodulation on the brain during aging has been studied and reviewed by many authors [64][65].
The heterogeneity of astrocytes is poorly understood and has not been sufficiently studied. The study of the different forms that astrocytes acquire to activate and their actions during the processes in different neurological diseases must be deepened and has not been sufficiently explained in this research. Nor has it been addressed whether it is possible to act therapeutically on the different types of astrocytes in neurological diseases.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24098434
References
- Vasile, F.; Dossi, E.; Rouach, N. Human astrocytes: Structure and functions in the healthy brain. Brain Struct. Funct. 2017, 2017222, 2017–2029.
- Verma, S.; Khurana, S.; Vats, A.; Sahu, B.; Ganguly, N.K.; Chakraborti, P.; Gourie-Devi, M.; Taneja, V. Neuromuscular Junction Dysfunction in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1502–1527.
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486.
- Araque, A.; Carmignoto, G.; Haydon, P.G. Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol. 2001, 63, 795–813.
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5.
- Wang, Q.; Kong, Y.; Wu, D.Y.; Liu, J.H.; Jie, W.; You, Q.L.; Huang, L.; Hu, J.; Chu, H.D.; Gao, F.; et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat. Commun. 2021, 12, 3321.
- Notter, T. Astrocytes in schizophrenia. Brain Neurosci. Adv. 2021, 27, 23982128211009148.
- Butt, A.M.; Rivera, A.D. Astrocytes in Bipolar Disorder. Adv. Neurobiol. 2021, 26, 95–113.
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287.
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63.
- Halassa, M.M.; Fellin, T.; Takano, H.; Dong, J.H.; Haydon, P.G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007b, 27, 6473–6477.
- de la Torre, J.C.; Olmo, A.D.; Valles, S. Can mild cognitive impairment be stabilized by showering brain mitochondria with laser photons? Neuropharmacology 2019, 171, 107841.
- Mapps, A.A.; Thomsen, M.B.; Boehm, E.; Zhao, H.; Hattar, S.; Kuruvilla, R. Diversity of satellite glia in sympathetic and sensory ganglia. Cell Rep. 2022, 38, 110328.
- Ledda, M.; Blum, E.; De Palo, S.; Hanani, M. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience 2009, 164, 1538–1545.
- Uta, D.; Kato, G.; Doi, A.; Andoh, T.; Kume, T.; Yoshimura, M.; Koga, K. Animal models of chronic pain increase spontaneous glutamatergic transmission in adult rat spinal dorsal horn in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019, 512, 352–359.
- Morris, G.; Stubbs, B.; Köhler, C.A.; Walder, K.; Slyepchenko, A.; Berk, M.; Carvalho, A.F. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med. Rev. 2018, 41, 255–265.
- Bortolon, C.; Macgregor, A.; Capdevielle, D.; Raffard, S. Apathy in schizophrenia: A review of neuropsychological and neuroanatomical studies. Neuropsychologia 2018, 118, 22–33.
- Caeiro, L.; Ferro, J.M.; Costa, J. Apathy secondary to stroke: A systematic review and meta-analysis. Cerebrovasc. Dis. 2013, 35, 23–39.
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49.
- Kiechle, T.; Dedeoglu, A.; Kubilus, J.; Kowall, N.W.; Beal, M.F.; Friedlander, R.M.; Hersch, S.M.; Ferrante, R.J. Cytochrome C and caspase-9 expression in Huntington’s disease. Neuromol. Med. 2002, 1, 183–195.
- Li, S.H.; Lam, S.; Cheng, A.L.; Li, X.J. Intranuclear huntingtin increases the expression of caspase-1 and induces apoptosis. Hum. Mol. Genet. 2000, 9, 2859–2867.
- Pasinelli, P.; Houseweart, M.K.; Brown, R.H.; Cleveland, D.W. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2000, 97, 13901–13906.
- Garden, G.A.; Budd, S.L.; Tsai, E.; Hanson, L.; Kaul, M.; D’Emilia, D.M.; Friedlander, R.M.; Yuan, J. Masliah, E.; Lipton, S.A. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 4015–4024.
- Re, D.B.; Le Verche, V.; Yu, C.; Amoroso, M.W.; Politi, K.A.; Phani, S.; Ikiz, B.; Hoffmann, L.; Koolen, M.; Nagata, T.; et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron 2014, 81, 1001–1008.
- Ito, Y.; Ofengeim, D.; Najafov, A.; Das, S.; Saberi, S.; Li, Y.; Hitomi, J.; Zhu, H.; Chen, H.; Mayo, L.; et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016, 353, 603–608.
- Picon, C.; Jayaraman, A.; James, R.; Beck, C.; Gallego, P.; Witte, M.E.; van Horssen, J.; Mazarakis, N.D.; Reynolds, R. Neuron-specific activation of necroptosis signaling in multiple sclerosis cortical grey matter. Acta Neuropathol. 2021, 141, 585–604.
- Levenson, R.W.; Sturm, V.E.; Haase, C.M. Emotional and behavioral symptoms in neurodegenerative disease: A model for studying the neural bases of psychopathology. Annu. Rev. Clin. Psychol. 2014, 10, 581–606.
- Pagonabarraga, J.; Kulisevsky, J.; Strafella, A.P.; Krack, P. Apathy in Parkinson’s disease: Clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015, 14, 518–531.
- Carnes-Vendrell, A.; Deus, J.; Molina-Seguin, J.; Pifarré, J.; Purroy, F. Depression and Apathy after Transient Ischemic Attack or Minor Stroke: Prevalence, Evolution and Predictors. Sci. Rep. 2019, 9, 16248.
- Cortes, N.; Andrade, V.; Maccioni, R.B. Behavioral and Neuropsychiatric Disorders in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 899–910.
- Christoforidou, E.; Joilin, G.; Hafezparast, M. Potential of activated microglia as a source of dysregulated extracellular microRNAs contributing to neurodegeneration in amyotrophic lateral sclerosis. J. Neuroinflamm. 2020, 17, 135.
- Takahashi, M.; Tabu, H.; Ozaki, A.; Hamano, T.; Takeshima, T. Antidepressants for Depression, Apathy, and Gait Instability in Parkinson’s disease: A Multicenter Randomized Study. Intern. Med. 2019, 58, 361–368.
- Halassa, M.M.; Florian, C.; Fellin, T.; Munoz, J.R.; Lee, S.Y.; Abel, T.; Haydon, P.G.; Frank, M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 2009, 61, 213–219.
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187.
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007.
- Haydon, P.G. Astrocytes and the modulation of sleep. Curr. Opin. Neurobiol. 2017, 44, 28–33.
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774.
- Morris, G.; Berk, M.; Galecki, P.; Walder, K.; Maes, M. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Mol. Neurobiol. 2016, 53, 1195–1219.
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269.
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636.
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97, 742–768.
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173.
- Liu, S.; Park, S.; Allington, G.; Prelli, F.; Sun, Y.; Martá-Ariza, M.; Scholtzova, H.; Biswas, G.; Brown, B.; Verghese, P.B.; et al. Targeting Apolipoprotein E/Amyloid β Binding by Peptoid CPO_Aβ17-21 P Ameliorates Alzheimer’s Disease Related Pathology and Cognitive Decline. Sci. Rep. 2017, 7, 8009.
- Afif, I.Y.; Manik, A.R.; Munthe, K.; Maula, M.I.; Ammarullah, M.I.; Jamari, J.; Winarni, T.I. Physiological Effect of Deep Pressure in Reducing Anxiety of Children with ASD during Traveling: A Public Transportation Setting. Bioengineering 2022, 9, 157.
- Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering 2022, 9, 48.
- Beckman, J.S.; Carson, M.; Smith, C.D.; Koppenol, W.H. ALS, SOD and peroxynitrite. Nature 1993, 364, 584.
- Chang, W.S.; Wang, Y.H.; Zhu, X.T.; Wu, C.J. Genome-Wide Profiling of miRNA and mRNA Expression in Alzheimer’s Disease. Medical science monitor. Int. Med. J. Exp. Clin. Res. 2017, 23, 2721–2731.
- Bhat, H.; Lang, K.S.; Hardt, C.; Lang, J. Interferon in the CNS. Neuro-Signals 2019, 27, 44–53.
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681.
- Pitteri, M.; Magliozzi, R.; Bajrami, A.; Camera, V.; Calabrese, M. Potential neuroprotective effect of Fingolimod in multiple sclerosis and its association with clinical variables. Expert Opin. Pharmacother. 2018, 19, 387–395.
- Wang, B.; Huang, X.; Pan, X.; Zhang, T.; Hou, C.; Su, W.J.; Liu, L.L.; Li, J.M.; Wang, Y.X. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav. Immun. 2020, 88, 132–143.
- Lacomblez, L.; Bensimon, G.; Douillet, P.; Doppler, V.; Salachas, F.; Meininger, V. Xaliproden in amyotrophic lateral sclerosis: Early clinical trials. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis. 2004, 5, 99–106.
- Lopez-Ramirez, M.A.; Lai, C.C.; Soliman, S.I.; Hale, P.; Pham, A.; Estrada, E.J.; McCurdy, S.; Girard, R.; Verma, R.; Moore, T.; et al. Astrocytes propel neurovascular dysfunction during cerebral cavernous malformation lesion formation. J. Clin. Investig. 2021, 131, e139570.
- Sweitzer, S.; De Leo, J. Propentofylline: Glial modulation, neuroprotection, and alleviation of chronic pain. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 235–250.
- Kasindi, A.; Fuchs, D.T.; Koronyo, Y.; Rentsendorj, A.; Black, K.L.; Koronyo-Hamaoui, M. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells 2022, 11, 1578.
- Zhang, X.; Chen, S.; Lu, K.; Wang, F.; Deng, J.; Xu, Z.; Wang, X.; Zhou, Q.; Le, W.; Zhao, Y. Verapamil Ameliorates Motor Neuron Degeneration and Improves Lifespan in the SOD1G93A Mouse Model of ALS by Enhancing Autophagic Flux. Aging Dis. 2019, 10, 1159–1173.
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of rapamycin on aging and age-related diseases-past and future. GeroScience 2021, 43, 1135–1158.
- Barakat, A.; Hamdy, M.M.; Elbadr, M.M. Uses of fluoxetine in nociceptive pain management: A literature overview. Eur. J. Pharmacol. 2018, 829, 12–25.
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557.
- Shao, Y.; Tan, B.; Shi, J.; Zhou, Q. Methotrexate induces astrocyte apoptosis by disrupting folate metabolism in the mouse juvenile central nervous system. Toxicol. Lett. 2019, 301, 146–156.
- La Maestra, S.; Frosina, G.; Micale, R.T.; D’Oria, C.; Garibaldi, S.; Daga, A.; Pulliero, A.; Izzotti, A. Brain microglia activation induced by intracranial administration of oligonucleotides and its pharmacological modulation. Drug Deliv. Transl. Res. 2018, 8, 1345–1354.
- Ebrahimi, F.; Koch, M.; Pieroh, P.; Ghadban, C.; Hobusch, C.; Bechmann, I.; Dehghani, F. Time dependent neuroprotection of mycophenolate mofetil: Effects on temporal dynamics in glial proliferation, apoptosis, and scar formation. J. Neuroinflamm. 2012, 9, 89.
- Nichols, N.R.; Zieba, M.; Bye, N. Do glucocorticoids contribute to brain aging?. Brain research. Brain Res. Rev. 2001, 37, 273–286.
- Cardoso, F.D.S.; Gonzalez-Lima, F.; Gomes da Silva, S. Photobiomodulation for the aging brain. Ageing Res. Rev. 2021, 70, 101415.
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2018, 17, 1003–1017.
This entry is offline, you can click here to edit this entry!
