Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microplastics (MPs), small plastic particles resulting from the degradation of larger plastic items and from primary sources such as textiles, engineered plastic pellets, etc., have become a ubiquitous environmental pollutant. As their prevalence in the natural environment grows, concerns about their potential impacts on human health have escalated.
- circulatory system
- exposure pathways
- health implications
- human body
- translocation mechanisms
- environmental contaminants
- plastic particles
- toxicological effects
1. Introduction
Production of plastics has significantly increased since they were originally developed in the 1950s [1]. The environment’s plastics, which frequently come from improperly disposed of consumer goods, slowly deteriorate due to photo and thermooxidative processes and, to a lesser extent by biodegradation. As a result, the material becomes less stable and breaks down into secondary MPs, which are fragments smaller than 5 mm [2]. According to Browne et al. [3], primary MPs are defined as plastic particles of this size that are intentionally produced for use in products (such as toothpaste or cosmetic exfoliants) or by companies (such as air blasting). MPs pollution has been noted as a rising worldwide problem over the past ten years that may have an impact on ecosystems, biodiversity, and human health [4]. They are persistent pollutants that have been identified as a developing global problem [5].
MPs have been found in a wide range of environments, including freshwater [6][7][8], seawater [9][10], sediment [11][12], soil [13][14], street dust [15], air [16][17], and even consumables such as beer, sea salt, and tap water [18]. Due to the significant deposits of MPs, the modern era has been referred to as “a new historical epoch, the Plasticene” [19]. Recent research has shown that MPs regularly enter the human body through ingestion, inhalation, and dermally, posing a serious threat to health [20]. According to Galloway [21], ingestion is the major route for entering MPs in the human body. The predicted intake of MPs is 39,000–52,000 particles per person per year based on food consumption [22]. Particles may enter the digestive tract through contaminated food or through mucociliary clearance following inhalation, which may trigger an inflammatory response, increase permeability, and alter the composition and metabolism of the gut microbes [23]. According to Prata [24], each person inhales 26–130 airborne MPs every day. A male person with light exercise is predicted to inhale 272 MPs each day based on air sampling using a mannequin [25][26]. The size and density of the particles will affect how they deposit on the respiratory system, with less dense and smaller particles penetrating the lungs deeper. Particle translocation may occur after deposition as a result of macrophage clearance, migration to the bloodstream, or lymphatic system. Dermal contact with MPs is considered a less significant route of exposure, although it has been speculated that nanoplastics (NPs <100 nm) could transverse the dermal barrier [27]. Human epithelial cells suffer oxidative stress from exposure to MPs and NPs as well [28]. Although research on the interactions between MPs and other human organs is ongoing, human absorption models of nanomaterials created by diverse industrial production methods can be used to estimate the potential impacts of MPs. They have been demonstrated to infiltrate the food chain [4], and as a result, they have also been found in human stools [29] and human blood of healthy donors [30]. Due to the prevalence of stool contamination after vaginal birth and investigated that human placenta from caesarean deliveries contains MPs (>50 µm).
From these pathways, MPs have recently been reported in unimaginable parts of the body and bodily fluid systems such as liver [31], blood [31][32], heart [32], placenta [33], breast milk [34], sputum [35], semen [36], testis [36], and urine [37]. Through various mechanisms, such as ingestion, inhalation, and dermal absorption, these tiny plastic particles have managed to enter people's bloodstream, lymphatic system, and organs. The implications of this phenomenon for human health are profound and multifaceted, as MPs may carry toxic additives, adsorb harmful chemicals, and trigger inflammatory responses. The researchers examines the pathways through which MPs enter the body, exploring the role of contaminated air, water, and food sources. It also investigates the extent to which MPs are capable of translocating within the body, potentially leading to accumulation in critical organs such as the liver, kidneys, and even the heart. Researchers are actively working to understand the long-term consequences of such internal plastic exposure, including the possible links to chronic diseases such as cancer, autoimmune disorders, and neurological conditions [31][32][33][34][35][36][37].
2. Plausible Pathways of MPs to Different Organs and Bodily Fluids
An overview of the plausible pathways of MPs to different organs and bodily fluids is presented in Figure 3. MPs may enter the circulation by a variety of routes, such as ingesting, inhaling, and cutaneous absorption. Once within the body, they may build up in the digestive system. Given that MPs are frequently present in seafood, drinking water, and even the air people breathe, studies indicate that ingestion is the primary method by which they enter the body [38]. Smaller particles can enter the bloodstream once they reach the gastrointestinal tract through: (1) permeability, where MPs may pass through the intestinal epithelium due to its porous nature, as MPs have been observed in human colectomy samples [39]; (2) MPs may be transported through the lymphatic system, which is involved in immune cell transport and the absorption of fats [40]; and (3) from the liver, which turns nitrogenous waste into urea, a less harmful compound. The circulation receives urea once liver cells release it [41]. As a result, MPs could enter the bloodstream. From the bloodstream, MPs can potentially be distributed to various organs and tissues, such as breast milk, heart, placenta, sputum, semen, testis and urine (Figure 1).
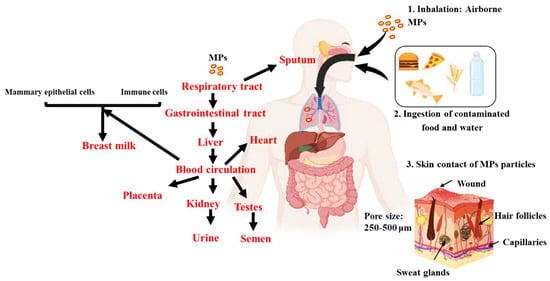
Figure 1. An overview of the plausible pathways of MPs to different organs and bodily fluids. Intake of MPs are possible via inhalation, ingestion and skin contact. Once in the body, they are absorbed into the gastrointestinal tract from which it is transported to the bloodstream. The bloodstream then circulates it to the different internal organs and then potentially excreted via the urine and semen.
According to one theory, MPs move from the circulation into breast milk via two different paths, each of which depends on immune cells and mammary epithelial cells, with the latter being particularly important for inhaled particles [34]. The major cells in charge of generating and secreting breast milk are mammary epithelial cells. These cells go through particular physiological changes during lactation in order to produce and transport different substances, such as proteins, lipids, and carbohydrates, into breast milk [42]. According to the theory, during this secretion phase MPs can be picked up by mammary epithelial cells and then released into the breast milk. One potential explanation is that MPs may be recognised by certain receptors on the surface of mammary epithelial cells once they have entered the circulation or interstitial fluid surrounding the mammary glands. When the MPs are taken up by vesicles and carried into the cytoplasm of the cell, a mechanism known as receptor-mediated endocytosis may be used by the cells to internalise the MPs [43]. Once within the cell, the MPs could be contained in milk secretory vesicles and discharged during lactation into the mother’s milk. Passive diffusion is another possible process. MPs could diffuse into the cells and then be delivered to milk secretory vesicles if their size and chemical makeup enable them to pass past the cellular membranes of mammary epithelial cells. The second route involves immune cells such as macrophages, which are found in breast tissue and assist in immunological defence by phagocytosing and eliminating foreign substances and debris from the lungs [44]. Including MPs, these foreign particles allow for engulfment and internalisation. After being taken up by immune cells in the lungs, MPs can then move via the lymphatic system or circulation until they reach the breast tissue, where they may be released into breast milk during lactation.
It is important to remember that the actual processes may be more complicated than those proposed, and that the concept of MPs translocating to breast milk through these routes is still an active area of research. The MPs’ precise dimensions, make-up, and interactions with diverse cells and tissues might have an impact on the translocation process. The quantity and presence of MPs in breast milk may also depend on additional variables such exposure levels, length of exposure, and individual variances.
In the case of the heart, it may take place as a result of MPs penetrating the blood channel endothelial cells. Additionally, MPs can cause oxidative stress and inflammation, which can cause the blood vessel barrier to rupture and let MPs into the heart tissue [32]. MPs can cross the placental barrier after entering the mother’s circulation and go to the foetal side [45]. During pregnancy, a unique structure called the placental barrier divides the maternal blood supply from the foetal blood supply [45]. Its primary job is to defend against hazardous chemicals while facilitating the flow of gases, nutrients, and other vital substances. Syncytiotrophoblast, the placenta’s outermost layer, foetal endothelial cells, and other supporting tissues make up the placental barrier’s many layers [46]. Theoretically, there are two ways that MPs might traverse the placental barrier: (1) through microscopic holes or breaches in the barrier, which may also be created by inflammation and barrier disruption; (2) by endocytosis, in which MPs are taken up by cells and transported over the barrier; and (3) immunological cells can also transfer MPs through the barrier as part of immunological reactions. To completely comprehend the methods and degree of microplastic transfer to the placenta, more studies are necessary.
However, factors such as their elimination by renal filtration or biliary excretion, or their deposit in organs such as the liver, spleen, or other fenestrated capillaries and sinusoids, dictate the fate of plastic particles inside the body [30]. Sputum can also be used for elimination in the respiratory system. Nitrogenous waste must first be changed by the liver into a less dangerous molecule before being released from liver cells into the circulation and finally to the kidney [41]. Although the renal excretion may be a viable pathway for the removal of MPs, it is known that the glomerular filtration barrier only permits the transit of particles with sizes of 10 nm. The mechanism for this may involve exocytosis and endocytosis close to the tubular epithelial cells after leaving the glomerulus via the efferent artery and entering the peritubular capillaries, before the MPs are excreted into the urine [37]. MPs can also pass through the renal tubule system, though this is less common [37].
As the testes have a rich blood supply, it is possible that some MPs circulating in the blood could reach the testicular tissue which include a barrier (blood-testis barrier) (Figure 2). The testes are protected by this specialized barrier, which also helps to maintain a stable internal environment for spermatogenesis and protects developing sperm from harmful substances in the blood. However, some studies have suggested that certain toxicants may be able to disturb this barrier [47] and thus form a pathway of MPs reaching the testes.
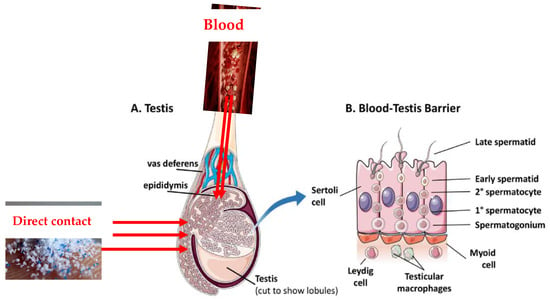
Figure 2. Plausible pathways of MPs into the testes. (A) is showing the specific location of the blood-testes barriers while (B) is unraveling the cellular composition and intricate structure of the blood-testis barrier, while exploring the supportive role of neighboring cells in maintaining barrier function and balance (Adapted with permission and modification from ref. [47]).
The lymphatic system is another potential pathway through which MPs could reach the testes. The lymphatic system is a network of vessels and organs that helps to transport lymph, a fluid containing white blood cells, throughout the body [48]. MPs may be taken up by immune cells in the lymphatic system and transported to different tissues, including the testes. However, in some cases, MPs may come into direct contact with the male reproductive system. For example, certain lifestyle or occupational exposures may lead to direct contact with MPs. Once MPs are in the testes, they might then find their way into the semen during the process of sperm maturation and secretion and through unknown mechanisms.
It is important to emphasize that the pathways described above are hypothetical and require further investigation and scientific evidence to be fully confirmed. As research progresses, it will be essential to explore the mechanisms by which MPs reach the semen and evaluate their potential impacts on male reproductive health. It is important to keep in mind that intrusive medical procedures might provide MPs direct access to the bloodstream and tissues. This is a potential source of contamination. There are MPs in the air in the operating theatre, according to recent research [49][50], which suggests that MPs may immediately descend to the surface of patients’ viscera through air [31]. It is necessary to look at these prospective sources’ roles more thoroughly.
This entry is adapted from the peer-reviewed paper 10.3390/environments10110194
References
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–Toxic Chemical Interaction: A Review Study on Quantified Levels, Mechanism and Implication. SN Appl. Sci. 2019, 1, 1400.
- Andrady, A.L. MPs in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605.
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179.
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348.
- United Nations Environment Programme. UNEP Year Book 2011: Emerging Issues in Our Global Environment; UNEP Publications: Nairobi, Kenya, 2011.
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic Pollution in the Surface Waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182.
- Estahbanati, S.; Fahrenfeld, N.L. Influence of Wastewater Treatment Plant Discharges on Microplastic Concentrations in Surface Water. Chemosphere 2016, 162, 277–284.
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and Temporal Distribution of MPs in Water and Sediments of a Freshwater System (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559.
- Norén Fredrik Small Plastic Particles in Coastal Swedish Waters. N-Research 2007, 11, 1–11.
- Mancuso, M.; Conti-Nibali, V.; Porcino, N. Monitoring of anthropogenic microplastic pollution in Antarctic fish (emerald rockcod) from the Terranova Bay after a quarter of century. Sci. Total Environ. 2023, 25, 167244.
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; Trigui El Menif, N. MPs in Sediments from the Littoral Zone of the North Tunisian Coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9.
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. MPs in Marine Sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463.
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended With Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2, 81.
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A Simple Method for the Extraction and Identification of Light Density MPs from Soil. Sci. Total Environ. 2018, 616–617, 1056–1065.
- Rabin, M.H.; Wang, Q.; Enyoh, C.E.; Kai, X.; Sheuty, T.F. Distribution, Potential Sources, and Health Risk of MPs (MPs) in Street Dust during and after COVID-19 Lockdown in Bangladesh. Environments 2023, 10, 130.
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and Potential Health Impacts of MPs and Microrubbers in Air and Street Dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164.
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of MPs in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293.
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970.
- Reed, C. Dawn of the Plasticene Age. New Sci. 2015, 225, 28–32.
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. MPs Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Health Toxicol. 2020, 35, e2020004.
- Galloway, T.S. Micro-and Nano-Plastics and Human Health. Mar. Anthropog. Litter 2015, 12, 343–366.
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of MPs. Environ. Sci. Technol. 2019, 53, 7068–7074.
- Salim, S.; Kaplan, G.; Madsen, K.L. Air Pollution Effects on the Gut Microbiota: A Link between Exposure and Inflammatory Disease. Gut Microbes 2013, 5, 215–219.
- Prata, J.C. Airborne MPs: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126.
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne MPs Using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670.
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne MPs: A Review Study on Method for Analysis, Occurrence, Movement and Risks. Environ. Monit. Assess. 2019, 191, 1–17.
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro (Nano) Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23.
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and MPs on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587.
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various MPs in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457.
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199.
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. MPs Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147.
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various MPs in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918.
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of MPs in Human Placenta. Environ. Int. 2021, 146, 106274.
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of MPs in Human Breastmilk. Polymers 2022, 14, 2700.
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of MPs in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486.
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of MPs in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713.
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of MPs in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2023, 11, 40.
- Shuaib Ibrahim, Y.; Tuan Anuar, S.; Azmi, A.A.; Mohd Afiq Wan Mohd Khalik, W.; Lehata, S.; Rabaah Hamzah, S.; Ismail, D.; Feei Ma, Z.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of MPs in Human Colectomy Specimens. JGH Open 2020, 5, 116–121.
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to MPs: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455.
- Watanabe, T. Physiological and Pathological Interactions between Liver and Kidney. Liver Syst. Dis. 2016, 221–249.
- McManaman, J.L. Lipid Transport in the Lactating Mammary Gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 35–42.
- Severin, E.S.; Posypanova, G.A. . Ross. Fiziol. Zh. Im. IM Sechenova 2011, 97, 553–565.
- Hallett, M.B. An Introduction to Phagocytosis. Adv. Exp. Med. Biol. 2020, 1246, 1–7.
- Zhu, D.; Gong, X.; Miao, L.; Fang, J.; Reports, J.Z.-S.C. Efficient Induction of Syncytiotrophoblast Layer II Cells from Trophoblast Stem Cells by Canonical Wnt Signaling Activation. Stem Cell Rep. 2017, 9, 2034–2049.
- Enyoh, C.E.; Duru, C.E.; Ovuoraye, P.E.; Wang, Q. Evaluation of NPs Toxicity to the Human Placenta in Systems. J. Hazard. Mater. 2023, 446, 130600.
- Yan Cheng, C.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64.
- Schindell, B.; Webb, A.; Kindrachuk, J. Persistence and Sexual Transmission of Filoviruses. Viruses 2018, 10, 683.
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B.; Fricker, G.; Deli, M.A. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921.
- Field, D.T.; Green, J.L.; Bennett, R.; Jenner, L.C.; Sadofsky, L.R.; Chapman, E.; Loubani, M.; Rotchell, J.M. MPs in the Surgical Environment. Environ. Int. 2022, 170, 107630.
- Petersen, E.J.; Ana, C.B.; Theodore, B.H.; Monique, E.J.; Albert, A.K.; Antonio, R.; Montoro, B.; Joanna, M.; Matthias, R.; Jian, Z.; et al. Potential Artifacts and Control Experiments in Toxicity Tests of Nanoplastic and Microplastic Particles. Environ. Sci. Technol. 2022, 56, 15192–15206.
This entry is offline, you can click here to edit this entry!
