1. Mechanism
Various levels of glucocorticoids have different effects on osteoblasts, osteoclasts, and osteocytes. Affected by excessive glucocorticoid usage, the most significant activities are the decrease in the proliferation of osteogenic precursors [
57] and suppression of osteoblast, reducing bone formation [
58]. However, the cellular and molecular mechanisms of glucocorticoids actions in bone remain not fully understood. With advanced technology, studies demonstrate a more detailed understanding on the mechanisms of glucocorticoids on bone turnover (
Figure 1).
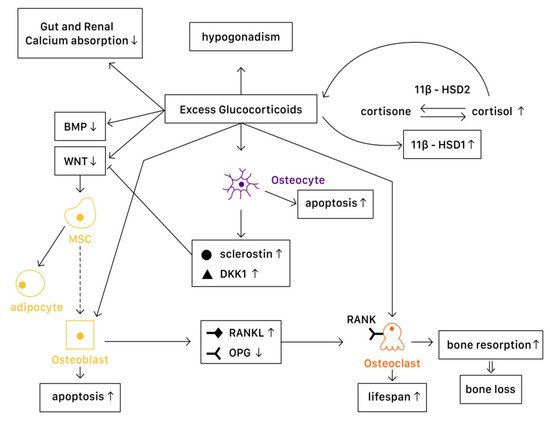
Figure 1. The mechanisms of glucocorticoid-induced osteoporosis (GIOP).
1.1. Physiology
Wnt signaling in osteoblasts and osteocytes promotes the production of osteoprotegerin (OPG), a cytokine receptor for NF-κB ligand (RANKL), thus inhibiting osteoclast formation and resulting in decreased bone resorption [
7]. Another important pathway is the bone morphogenetic protein (BMP) signaling pathway. Canonical BMP signaling requires the phosphorylation of Smad to activate, which controls the mesenchymal precursor cell’s differentiation, promoting osteoblast differentiation and bone formation [
59].
1.2. Pathophysiology
In Osteoblasts
In a pathophysiological state, excessive glucocorticoids inhibit Wnt protein production, causing mesenchymal progenitor cells to differentiate toward adipocytes rather than osteoblasts. On the other hand, excessive glucocorticoids also interfere with the canonical BMP pathway. One study suggested that in GIOP patients, casein kinase-2 interacting protein-1 (CKIP-1) mRNA and protein expression were both remarkably higher in bone, thus suppressing the Smad-dependent BMP signaling by promoting Smad1 ubiquitination and resulting in the inhibition of osteogenic differentiation [
59]. Another study revealed that MiR-106b, a type of miRNA transcribed from the miR-106b-25 cluster, inhibited osteoblastic differentiation of mesenchymal progenitor cells in vitro. In a GIOP mice model, Mi-106b increased its expression and inhibited bone formation partly through targeting BMP2 [
60]. Moreover, excess glucocorticoids are associated with osteoblast and osteocyte apoptosis, resulting in declined bone formation [
7]. Disruption of canopies that covers the bone remodeling surface was also recognized, leading to insufficient osteoblast recruitment [
61].
11β-Hydroxysteroid dehydrogenases (11β-HSDs), two isoenzymes that regulate glucocorticoid activity, also influence the effects of glucocorticoids on bone [
7,
58]. 11β-HSD type 1 (11β-HSD1) catalyzes the formation of active cortisol, and 11β-HSD type 2 (11β-HSD2) catalyzes the conversion of active glucocorticoids to inactive cortisone. Glucocorticoids are found to enhance 11β-HSD1 expression and activity in osteoblasts, and such enhancement could result in the amplification of the cellular actions of glucocorticoids [
58]. Not only in the cellular actions, dysregulation of 11β-HSDs is associated with renal calcium loss in primary male osteoporosis [
62], which may also contribute to bone loss.
In Osteoclasts
Excessive glucocorticoids are found to increase RANKL/OPG ratio, which promotes bone resorption by enhancing the maturation and activation of osteoclast. Lekva et al. demonstrated that glucocorticoids increased leucine zipper gene (GILZ) expression, which modulated the expression of OPG [
63]. With the reduction of OPG expression, osteoclastogenesis is favored. Additionally, the increase of IL-6 and other cytokines also enhance bone resorption, resulting in bone loss. The lifespan of osteoclast is also prolonged directly by glucocorticoids [
7].
In Osteocytes
Osteocytes have an important role in bone remodeling, which is dependent on mechanical and hormone signaling. As mentioned above, glucocorticoids are related to osteocyte apoptosis, which reduces bone integrity and increases the risk of fracture. Moreover, excess glucocorticoids induce production of sclerostin and Dickkopf-related protein 1 (DKK1) by osteocytes, thus suppressing the Wnt pathway. Through the activation of PPARγ, mesenchymal progenitors differentiate into adipocytes rather than osteoblasts [
7].
Systemic Effects
The mechanisms described above are the direct molecular signaling pathway of glucocorticoid actions. However, when glucocorticoids are given at higher doses, systemic effects cannot be overlooked. The well-documented effects include reductions in calcium absorption in both the gut and the renal tubule that can affect bone mineralization [
64]. Hypogonadism in males and premenopausal females is also recognized in high-dose glucocorticoids use, resulting in bone loss [
65]. Additionally, avascular necrosis via an apoptotic mechanism of osteocytes and osteoblasts [
66] and glucocorticoid-induced myopathy is considered to affect bone health and increase in falling risks [
7]. Finally, glucocorticoids alter metabolites and lipid profiles, which potentially have a role in regulating bone loss [
67,
68].
2. Assessment and Screening
According to the 2017 ACR guideline, initial clinical fracture risk assessment should be performed at least within 6 months of the initiation of glucocorticoid treatment. For adults over 40 years old, Fracture Risk Assessment Tool (FRAX) with glucocorticoid dose correction and bone mineral density (BMD) testing should be carried out as soon as possible for estimating the absolute fracture risk. As for adults below 40 years old, BMD testing should be carried out if high fracture risks are identified, such as a history of osteoporotic fracture or other significant osteoporotic risk factor [
10]. Other than BMD, trabecular bone score (TBS) is a novel analytical tool which can extract data from the two-dimensional lumbar spine dual-energy X-ray absorptiometry (DXA) image. It can provide information relating to trabecular microarchitecture, and is thus capable of greater discriminative power than BMD alone for fracture risk assessment in glucocorticoid-treated patients [
69]. For the threshold of therapeutic intervention, individual intervention threshold based on FRAX probability and a higher BMD cut-off point are recommended for GIOP [
70,
71].
Advanced bone imaging techniques have also been developed, providing structural information beyond BMD with superior discrimination of fracture in GIOP patients [
72]. Advanced methods for assessing macrostructure of bone include volumetric Quantitative Computed Tomography (vQCT), peripheral Quantitative Computed Tomography (pQCT), high-resolution Computed Tomography (hrCT), and high-resolution Magnetic Rresonance Imaging (hrMRI) [
73]. Although these advanced imaging techniques seem quite promising in the future, several clinical challenges remain, such as cost, radiation exposure, and complexity, which need further exploration.
3. Treatment
All adults who are under long-term glucocorticoid therapy should receive adequate intervention on preventing or minimizing GIOP. Unfortunately, a large proportion of patients are neither investigated properly nor treated according to the recommendations [
18,
74,
75]. Nonetheless, pharmacological treatments are at an even lower rate compared with non-pharmacological treatments [
76,
77].
3.1. Non-Pharmacological Treatment
Due to the interference of intestinal calcium absorption and hypercalciuria by glucocorticoids, vitamin D and calcium supplement are recommended [
78]. According to the 2017 ACR guideline, all adults taking prednisolone at a dose of over 2.5 mg/day for over 3 months should receive vitamin D (600–800 IU/day) and a calcium supplement (1000–1200 mg/day) [
10]. Life modifications are also important for bone health during the treatment.
3.2. Pharmacological Treatments
Bisphosphonate
Bisphosphonates are the most widely used first-line agents for prevention and treatment in GIOP [
79]. Studies have proven that bisphosphonates significantly increase BMD of the lumbar spine, total hip, and femoral neck in patients with GIOP [
80,
81]. Reducing the risk of vertebral fractures is also identified in patients treated with bisphosphonates in several studies [
82,
83]. The results of Positron Emission Tomography (PET) scan suggest that bisphosphonate has a direct effect on decreasing bone turnover, resulting in increased BMD [
84]. Urinary deoxypyridinoline (DPD), a marker of bone resorption, also decreases after bisphosphonate use [
85]. Some common oral forms of bisphosphonate agents include alendronate, etidronate, and risedronate. Injected bisphosphonate medication, such as zoledronic acid, is also available.
For moderate and high risks of major fracture patients, oral bisphosphonate is recommended over calcium and vitamin D alone [
10,
86]. Oral bisphosphonate (e.g., risedronate) is also recommended as initial treatment over IV bisphosphonate (e.g., zoledronic acid) due to its safety and cost in adults [
10]. Although a study of comparing zoledronic acid with risedronate in GIOP males showed significantly greater increases in lumbar spine BMD and total hip BMD in the zoledronic acid group, further safety evaluation is needed for IV bisphosphonate [
87]. A combination therapy of plastrum testudinis extract and alendronate in rat spine has shown synergic effects on preventing GIOP; however, more studies should be conducted for further confirmation and application [
88].
Calcitonin
Calcitonin is a hormonal agent that inhibits bone resorption, with both injected and inhaled forms available. According to previous studies, calcitonin increases BMD in GIOP; however, there is no effect shown on fracture reduction [
83,
89,
90]. Due to its modest efficacy and less effective actions than bisphosphonate, it is not considered as a first-line treatment of GIOP [
89,
91].
Hormone Replacement Therapy (HRT)
Hypogonadism is a side effect of long term corticosteroid use; thus, hormone replacement therapy (HRT) is considered as a second-line or third-line treatment in GIOP patients with sex hormone deficiency [
7]. Estrogen has been shown to increase BMD in GIOP patients with sex hormone deficiency [
92]; however, increased risks of cardiovascular diseases and breast cancer have significantly reduced its bone benefit. Alternatively, testosterone in males with hypogonadism has been shown to prevent bone loss at the lumbar spine in GIOP [
92,
93]. On the other hand, the selective estrogen-receptor modulator (SERM) raloxifene also reduces fracture occurrence in postmenopausal females [
7,
90]. Due to a lack of adequate data and potential harms, raloxifene is only conditionally recommended in postmenopausal females who are not appropriate for any other GIOP medications [
10].
Teriparatide
Teriparatide is a human recombinant parathyroid hormone (PTH) that increases BMD and reduces the risk of fracture. It activates osteoblasts, which subsequently lead to an increase in bone growth. Teriparatide can also reduce the cellular ROS level elevated by the actions of glucocorticoids to facilitate the proliferation of osteocytes [
94].
One randomized controlled trial showed significantly greater increases in spine and hip BMD in the teriparatide group compared with the alendronate group during a 36-months therapy [
95]. Increases in biomarkers of bone formation in the teriparatide group with decreases in biomarkers in the alendronate group were also noted [
95,
96,
97]. Along with the bone effects of teriparatide, the decrease in serum HbA1c with some improvement in glucose homeostasis in patients with diabetes and GIOP was recognized among teriparatide users [
98]. Despite the fact that many studies have shown teriparatide is more clinically efficacious than bisphosphonates for GIOP, cost burden is still an important issue to be solved [
99]. For the time being, bisphosphonate remains the first-line treatment due to its cost effectiveness [
10].
Denosumab
Denosumab is a RANKL inhibitor that acts similarly as endogenous osteoprotegerin, which reduces bone loss and increases bone density. One randomized, active-controlled trial revealed that denosumab was superior in increasing BMD in lumbar spine and total hip compared with risedronate [
100]. The combination therapy of denosumab and teriparatide in a mouse model was shown to increase BMD in the lumbar spine and regenerated cancellous bone volume compared with a single administration of each agent [
101]. In spite of the promising future of denosumab in treating GIOP, more safety data in patients under an immunosuppressant status need to be collected in the future.
Strontium Ranelate
As an alternative medication for osteoporosis, strontium ranelate consists of strontium salts of ranelic acid, and acts to increase BMD by both enhancing deposition of new bone by osteoblasts and reducing the resorption of bone by osteoclasts. Because the nucleus of strontium is almost the same size as that of calcium, it is easily incorporated into bones to increase bone formation. Furthermore, strontium ranelate promotes the differentiation of pre-osteoblasts to osteoblasts and stimulates osteoblasts to produce osteoprotegerin, which can block the action of RANKL to inhibit the differentiation of preosteoclasts to osteoclasts. In clinical practice, it can significantly lower the risks of vertebral and hip fractures when compared with placebo. However, the serous side effect of strontium ranelate has largely limited its use because of an increased risk of myocardial infarction noted in randomized trials. Currently, the clinical use is restricted to the treatment of severe osteoporosis in postmenopausal females who are at high risk for fractures.
Future Possible Treatments
Several experiments targeting on different GIOP pathways were conducted. One study revealed that alpha-lipoic acid, an endogenous anti-oxidant, had a positive effect on improving osteopenia by inhibiting oxidative stress and apoptosis in rat models [
102]. Another study targeting on sialic acid-binding immunoglobulin-like lectin 15 (Siglec-15), an immunomoderator that regulated terminal differentiation of osteoclasts, showed promising results on protective effects against glucocorticoid-induced bone loss by suppressing bone resorption in anti-Siglec-15 therapy [
103]. On the other hand, herbal medications such as red ginseng, chicory, and curcumin might also have protective effects on GIOP [
104,
105,
106]. However, their effects remain to be investigated due to the small sample size of the existent studies.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031376