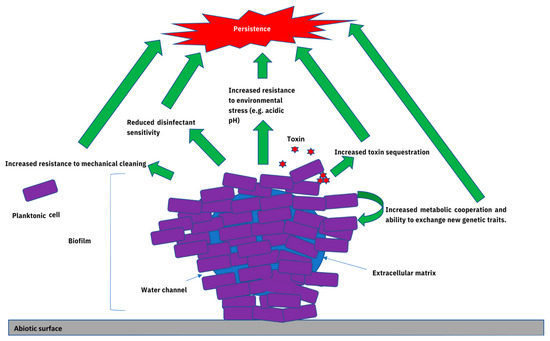
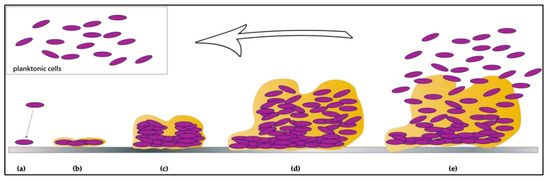
Non-typhoidal
S. enterica is a Gram-negative bacillus and a common cause of foodborne gastroenteritis, usually associated with poultry products [
78]. However, contaminated meat, fresh produce, nuts, spices, flour, milk and drinking water can also act as vehicles [
78]. Like LM,
S. enterica is able to form biofilms on a range of surface materials and to persist in food-associated environments, such as poultry slaughterhouses [
79]. Vestby et al. [
74] reported that the biofilm-forming ability of 111
Salmonella strains isolated from feed and fish meal factories was correlated with their persistence within the factory environment.
E-coli is a Gram-negative bacillus, which is closely related to
S. enterica [
78]. While most strains are non-pathogenic, some are highly pathogenic. For example, the Shiga-toxin-producing strain
E-coli O157:H7 can cause haemorrhagic colitis and potentially life-threatening haemolytic uraemic syndrome [
78].
E-coli outbreaks are associated with the consumption of raw meat or meat products, as well as fresh produce [
78]. The organism was found to persist on a conveyor belt in a meat packing plant, surviving daily cleaning and disinfection practices [
83]. Beef-production companies have also been known to experience periods of increased product contamination caused by
E-coli O157:H7 [
76], most likely due to persistent contamination of the processing environment [
84].
Campylobacter spp. are Gram-negative, microaerophilic bacilli [
82]. They are the common cause of bacterial gastroenteritis globally, particularly
C. jejuni and
C. coli [
86]. Like
Salmonella, infection is usually associated with poultry meat, as well as other foods, such as raw milk [
82]. The organism requires specific conditions to grow, such as a microaerophilic atmosphere and a temperature between 37 °C and 42 °C [
82]. It is also sensitive to food-processing conditions, such as acidic pH [
82]. Despite this, there is evidence that it can persist within food-associated environments, such as farms and dairies, by forming biofilms [
86].
4. Phenotypic Comparisons of Biofilm Development in Persistent and PNP LM Strains
Several studies have reported evidence of enhanced biofilm formation among persistent LM strains [19,27,29,40,87,88,89,90,91]. For example, Norwood and Gilmour [27] measured the adherence to stainless steel coupons of 32 persistent and 25 PNP strains isolated from farms and food processing plants. After 24 h of surface contact at 25 °C, mean plate counts on the coupons were significantly higher for the persistent strains. Similar results were obtained by Lunden et al. [87] after they compared the adherence of a persistent strain to that of three PNP strains isolated from meat dicing plants on stainless steel coupons over two hours at 25 °C using epifluorescence microscopy counts.
A similar number of studies have reported no apparent difference between the biofilm formation of persistent and PNP LM strains [47,56,92,93,94,95,96,97,98,99,100,101]. For example, Djordjevic et al. [56] compared the biofilm formation of five persistent and five PNP strains isolated from fish processing plants to PVC microtiter plates using the crystal-violet method; after 40 h of surface contact at 32 °C, no relationship was observed between the biofilm levels of the persistent and PNP strains.
Factors that may explain the inconsistent findings between studies could include differences in experimental conditions, such as incubation temperature, surface material and nutrient availability [1,13,16,41].
The biofilm formation of persistent LM strains has also been found to vary according to the composition and concentration of the growth medium [19,44,103] and the surface material [104]. For example, Lee et al. [44] found that persistent strains exhibited significantly increased biofilm formation compared with PNP strains, but only in neat (undiluted), as opposed to dilute (1:10), brain heart infusion (BHI) broth.
In addition to different experimental conditions, there are several other factors that might account for the conflicting results between studies investigating the relationship between biofilm formation and persistence. These include differences in the choice of biofilm assay, sample size, persistence definitions, and/or test strain properties, such as lineage or serotype [4,29]. Differences in biofilm formation according to serotype have been reported in E-coli, with serotype O103:H2 isolates shown to produce more biofilm at 12 °C, 20 °C and 37 °C, compared with strains representative of serotypes O26:H11 or O103:H25 [105].
5. Genetic Mechanisms Involved in Biofilm Development in LM
With advances in WGS techniques, a few studies have compared the genomes of persistent and PNP LM strains to identify genetic markers that may help to explain, identify or predict persistence. This includes markers associated with biofilm production (summarised in
Table S1). The following section provides an overview of the functions of some of the main genetic loci associated with biofilm formation in LM, which have been investigated for an association with persistence in WGS studies, before going on to discuss the key findings of these studies.
One of the main biofilm-associated genes in LM is
flaA, which encodes the main structural component of the bacterial flagella, flagellin A [
113]. Flagella-mediated motility appears to promote initial attachment under both static and dynamic conditions but is not essential for—and may even inhibit—full biofilm formation under dynamic conditions [
114,
115,
116,
117]. Expression of
flaA in LM is controlled by at least five other regulatory genes [
113]. Some of these genes have also been shown to be required for effective biofilm development, which may be due, in part, to their effect on flagella expression and motility. These include the positive regulatory factor A gene,
prfA, involved in the later stages of biofilm development [
118,
119], and the degradation enzyme regulator gene (
degU), involved in adherence to plastic surfaces [
120,
121].
The actin-assembly-inducing peptide precursor (ActA), encoded by
actA, is another important virulence determinant in LM, which promotes bacterial aggregation and biofilm development [
122]. Compared with wild-type (WT) strains, Δ
actA mutant strains exhibit significant reductions in biofilm development on glass under both static and dynamic conditions due to reduced clustering. The transcriptional regulator of stress response genes, SigB, is also required to reach WT biofilm levels under both static and continuous-flow conditions on stainless steel and polystyrene [
123,
124]. However, it may only be involved in the later stages of biofilm development, as no difference in biofilm levels was observed between WT and SigB-deficient strains after 24–40 h of surface contact [
116,
123]. Contrastingly, the biofilm-associated protein (BapL), encoded by
bapL, appears to be involved in adherence in some LM strains, but its exact role in biofilm development is not well understood [
113,
125].
The listerial virulence proteins internalins A and B (InlA and InlB) have also been implicated in listerial biofilm development [
125]. Deletion of
inlA or
inlB in LM EGD-e has been associated with significant reductions in adherence to glass compared with a WT strain, especially when both genes are deleted [
127,
128]. However,
inlA truncation has been associated with increased biofilm production compared with WT strains [
129]. The truncated protein lacks the cell wall-binding domain and is secreted where it undergoes proteolytic cleavage and may contribute towards the biofilm EM. Internalin L (InlL) appears to play a similar role in listerial adherence, as deletion of
inlL has been associated with reduced attachment to polystyrene [
130].
The
agrBDCA operon is a peptide-based QS system in LM that plays an important role in biofilm development [
131,
132,
133]. It consists of four genes, including
agrC,
agrA,
agrD and
agrB, respectively encoding a histidine kinase (AgrC), a response regulator (AgrA), a precursor peptide (AgrD) and a protein (AgrB), which converts the precursor peptide into an autoinducing peptide. LM Δ
agrA and Δ
agrD deletion mutants both exhibit significant reductions in adherence to glass or polystyrene surfaces compared with WT strains within the first 24 h of surface contact [
131,
132].
The LuxS system is another QS system in LM that has been implicated in biofilm development [
11,
113]. It encodes a protein (LuxS) involved in producing autoinducer-2 (AI-2) precursor molecules [
55]. LM strains with mutations in
luxS have been known to produce denser biofilms and to attach more readily to glass compared with WT strains [
135,
136].
6. WGS Comparisons of Biofilm Production in Persistent and PNP LM Strains
One of the most comprehensive attempts to identify persistence markers was conducted by Nielsen et al. [
140]. Based on a literature search, the authors identified 15 genes which might play a role in persistence. Six of them were chosen due to evidence of involvement in biofilm production, including
actA,
bapL,
recO,
lmo2504,
luxS and the flagellar operon gene,
lmo0673. They used WGS to compare the genomes of 21 persistent and 17 PNP strains isolated from pork and salmon-processing plants to see whether any of these genes were overrepresented among persistent strains. However, none of the genes examined were clearly linked to persistence, with equal representation among persistent and PNP isolates. The genes were also examined for any insertions, deletions or single nucleotide polymorphisms (SNPs) that might distinguish persistent LM strains, but none were identified. Similar results were reported by Muhterem-Uyar et al. [
141], who examined the genomes of six persistent strains and five PNP strains isolated from an Austrian cheese processing plant for the presence of genes associated with survival in food-associated environments. Twenty-eight of them were genes associated with biofilm development, including
lmo2504,
flaA,
agrD,
agrA,
degU,
luxS,
bapL and
agrA (among others). However, these genes were largely present in both persistent and PNP strains.
Overall, WGS studies have failed to identify any consistent genetic differences between persistent and PNP LM strains linked to biofilm production. This would appear to support earlier suggestions that persistence may be due to a failure of cleaning and sanitation procedures to remove LM from harbourage sites in food-associated environments, as opposed to any unique strain properties [
17,
18]. While environmental factors such as this may significantly contribute to persistence, this is not to say that some strains do not still harbour traits that increase their chances of persisting within food-associated environments [
18,
99]. Enhanced biofilm production may be just one of these, the importance of which may depend on the strain or environmental conditions or a complex interplay between the two. This may explain why some studies focusing on one trait, such as enhanced biofilm formation or disinfectant tolerance, have observed an association with persistence but not others [
99].
7. Conclusions
Overall, it remains unclear as to whether enhanced biofilm formation contributes towards listerial persistence. While numerous phenotypic studies have been carried out to investigate this, the results have been highly conflicting. WGS studies, while limited in number, have also failed to identify any biofilm markers clearly linked to persistence. This may have been due, in part, to authors having relied upon different persistence definitions and/or different experimental protocols. Alternatively, it may be that biofilm formation is just one of several mechanisms that LM uses to persist, the importance of which may depend upon the strain, environmental conditions, or a complex interplay between the two. Clearly, a greater understanding of the mechanisms that LM uses to persist in food-associated environments is needed. However, for this to be achieved, further research is needed.