Cell survival, homeostasis and cell polarity rely on the control of membrane trafficking pathways. The RUN domain (comprised of the RPIP8, UNC-14, and NESCA proteins) has been suggested to be implicated in small GTPase-mediated membrane trafficking and cell polarity. Accumulating evidence supports the hypothesis that the RUN domain-containing proteins might be responsible for an interaction with a filamentous network linked to actin cytoskeleton and/or microtubules. In addition, several downstream molecules of PI3K are involved in regulation of the membrane trafficking by interacting with vesicle-associated RUN proteins such as RUFY family proteins.
- RUFY family
- RUN domain
- membrane trafficking
- small GTPase
- NESCA
- Rab
- Rap
1. Introduction
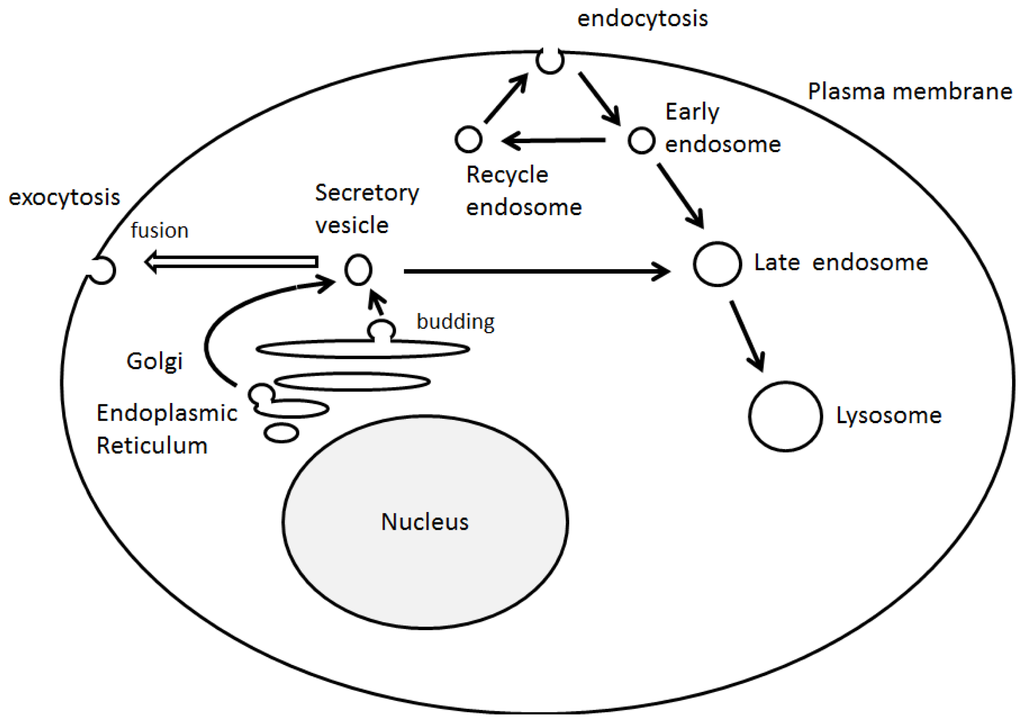
2. RUN Domain Binds Several Signaling Molecules
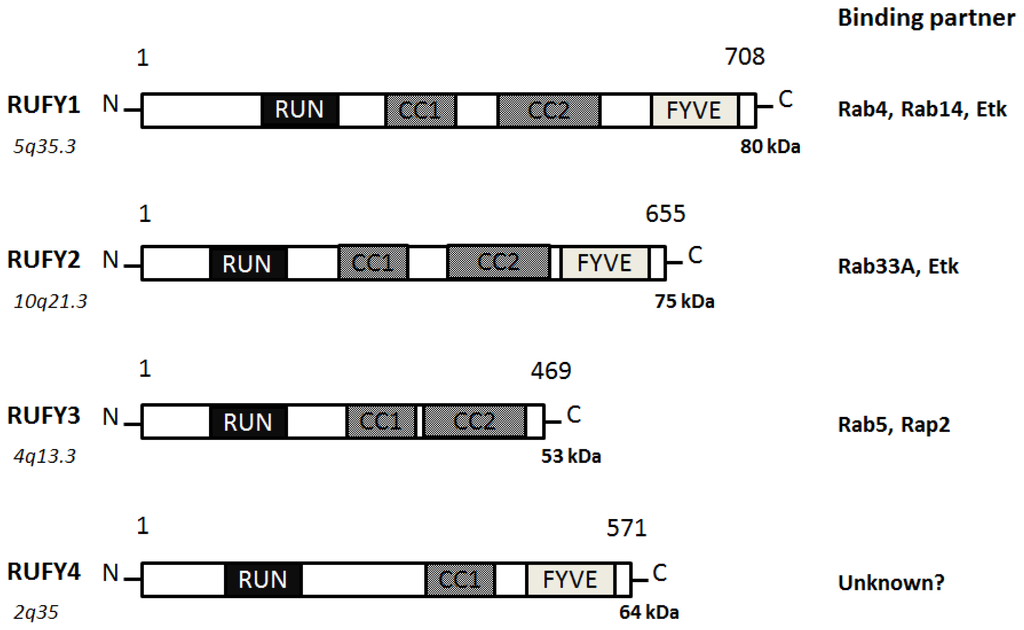
3. Function of RUFY Family Proteins with the RUN Domain
4. Regulation of Cell Polarity and Membrane Trafficking
This entry is adapted from the peer-reviewed paper 10.3390/ijms14036487
References
- Happé, H.; de Heer, E.; Peters, D.J. Polycystic kidney disease: The complexity of planar cell polarity and signaling during tissue regeneration and cyst formation. Biochim. Biophys. Acta 2011, 1812, 1249–1255.
- Santiago-Tirado, F.H.; Bretscher, A. Membrane-trafficking sorting hubs: Cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol 2011, 21, 515–525.
- Baum, B.; Georgiou, M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol 2011, 192, 907–917.
- Wu, G.; Ge, J.; Huang, X.; Hua, Y.; Mu, D. Planar cell polarity signaling pathway in congenital heart diseases. J. Biomed. Biotechnol 2011, 2011, 589414.
- Traub, L.M. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol 2009, 10, 583–596.
- Martin-Belmonte, F.; Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 2011, 12, 23–38.
- Layton, A.; Savage, N.S.; Howell, A.S.; Carroll, S.Y.; Drubin, D.G.; Lew, D.J. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr. Biol 2011, 21, 184–194.
- Golachowska, M.R.; Hoekstra, D.; van IJzendoorn, S.C. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol 2010, 20, 618–626.
- Callebaut, I.; de Gunzburg, J.; Goud, B.; Mornon, J.P. RUN domains: A new family of domains involved in Ras-like GTPase signaling. Trends Biochem. Sci 2001, 26, 79–83.
- MacDonald, J.I.; Kubu, C.J.; Meakin, S.O. Nesca, a novel adapter, translocates to the nuclear envelope and regulates neurotrophin-induced neurite outgrowth. Cell Biol 2004, 164, 851–862.
- Yoshida, H.; Kitagishi, Y.; Okumura, N.; Murakami, M.; Nishimura, Y.; Matsuda, S. How do you RUN on? FEBS Lett 2011, 585, 1707–1710.
- Kukimoto-Niino, M.; Takagi, T.; Akasaka, R.; Murayama, K.; Uchikubo-Kamo, T.; Terada, T.; Inoue, M.; Watanabe, S.; Tanaka, A.; Hayashizaki, Y.; et al. Crystal structure of the RUN domain of the RAP2-interacting protein x. J. Biol. Chem 2006, 281, 31843–31853.
- Sun, Q.; Han, C.; Liu, L.; Wang, Y.; Deng, H.; Bai, L.; Jiang, T. Crystal structure and functional implication of the RUN domain of human NESCA. Protein Cell 2012, 3, 609–617.
- Pankiv, S.; Alemu, E.A.; Brech, A.; Bruun, J.A.; Lamark, T.; Overvatn, A.; Bjørkøy, G.; Johansen, T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol 2010, 188, 253–269.
- Sakamoto, R.; Byrd, D.T.; Brown, H.M.; Hisamoto, N.; Matsumoto, K.; Jin, Y. The Caenorhabditis elegans UNC-14 RUN domain protein binds to the kinesin-1 and UNC-16 complex and regulates synaptic vesicle localization. Mol. Biol. Cell 2005, 16, 483–496.
- Ogura, K.; Goshima, Y. The autophagy-related kinase UNC-51 and its binding partner UNC-14 regulate the subcellular localization of the Netrin receptor UNC-5 in Caenorhabditis elegans. Development 2006, 133, 3441–3450.
- Yang, J.; Kim, O.; Wu, J.; Qiu, Y. Interaction between tyrosine kinase Etk and a RUN domain- and FYVE domain-containing protein RUFY1. A possible role of ETK in regulation of vesicle trafficking. J. Biol. Chem 2002, 277, 30219–30226.
- Larance, M.; Ramm, G.; Stöckli, J.; van Dam, E.M.; Winata, S.; Wasinger, V.; Simpson, F.; Graham, M.; Junutula, J.R.; Guilhaus, M.; James, D.E. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem 2005, 280, 37803–37813.
- Fouraux, M.A.; Deneka, M.; Ivan, V.; van der Heijden, A.; Raymackers, J.; van Suylekom, D.; van Venrooij, W.J.; van der Sluijs, P.; Pruijn, G.J. Rabip4′ is an effector of rab5 and rab4 and regulates transport through early endosomes. Mol. Biol. Cell 2004, 15, 611–624.
- Simonsen, A.; Wurmser, A.E.; Emr, S.D.; Stenmark, H. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol 2001, 13, 485–492.
- Mari, M.; Macia, E.; le Marchand-Brustel, Y.; Cormont, M. Role of the FYVE finger and the RUN domain for the subcellular localization of Rabip4. J. Biol. Chem 2001, 276, 42501–42508.
- Yamamoto, H.; Koga, H.; Katoh, Y.; Takahashi, S.; Nakayama, K.; Shin, H.W. Functional cross-talk between Rab14 and Rab4 through a dual effector, RUFY1/Rabip4. Mol. Biol. Cell 2010, 21, 2746–2755.
- Mari, M.; Monzo, P.; Kaddai, V.; Keslair, F.; Gonzalez, T.; le Marchand-Brustel, Y.; Cormont, M. The Rab4 effector Rabip4 plays a role in the endocytotic trafficking of Glut 4 in 3T3-L1 adipocytes. J. Cell Sci 2006, 119, 1297–1306.
- Cormont, M.; Mari, M.; Galmiche, A.; Hofman, P.; le Marchand-Brustel, Y. A FYVE-finger-containing protein, Rabip4, is a Rab4 effector involved in early endosomal traffic. Proc. Natl. Acad. Sci. USA 2001, 98, 1637–1642.
- Barbe, L.; Lundberg, E.; Oksvold, P.; Stenius, A.; Lewin, E.; Björling, E.; Asplund, A.; Pontén, F.; Brismar, H.; Uhlén, M.; Andersson-Svahn, H. Toward a confocal subcellular atlas of the human proteome. Mol. Cell Proteomics 2008, 7, 499–508.
- Fukuda, M.; Kobayashi, H.; Ishibashi, K.; Ohbayashi, N. Genome-wide investigation of the Rab binding activity of RUN domains: Development of a novel tool that specifically traps GTP-Rab35. Cell Struct. Funct 2011, 36, 155–170.
- Mori, T.; Wada, T.; Suzuki, T.; Kubota, Y.; Inagaki, N. Singar1, a novel RUN domain-containing protein, suppresses formation of surplus axons for neuronal polarity. J. Biol. Chem 2007, 282, 19884–19893.
- Yoshida, H.; Okumura, N.; Kitagishi, Y.; Shirafuji, N.; Matsuda, S. Rab5(Q79L) interacts with the carboxyl terminus of RUFY3. Int. J. Biol. Sci 2010, 6, 187–189.
- Hammad, S.M.; Twal, W.O.; Barth, J.L.; Smith, K.J.; Saad, A.F.; Virella, G.; Argraves, W.S.; Lopes-Virella, M.F. Oxidized LDL immune complexes and oxidized LDL differentially affect the expression of genes involved with inflammation and survival in human U937 monocytic cells. Atherosclerosis 2009, 202, 394–404.
- Kimura, K.; Wakamatsu, A.; Suzuki, Y.; Ota, T.; Nishikawa, T.; Yamashita, R.; Yamamoto, J.; Sekine, M.; Tsuritani, K.; Wakaguri, H.; et al. Diversification of transcriptional modulation: Large-scale identification and characterization of putative alternative promoters of human genes. Genome Res 2006, 16, 55–65.
- Pfeffer, S.R. Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett 2009, 583, 3811–3816.
- González-Gaitán, M. Endocytic trafficking during Drosophila development. Mech. Dev 2003, 120, 1265–1282.
- Bakhru, S.H.; Altiok, E.; Highley, C.; Delubac, D.; Suhan, J.; Hitchens, T.K.; Ho, C.; Zappe, S. Enhanced cellular uptake and long-term retention of chitosan-modified iron-oxide nanoparticles for MRI-based cell tracking. Int. J. Nanomed 2012, 7, 4613–4623.
- Monck, J.R.; Fernandez, J.M. The exocytotic fusion pore and neurotransmitter release. Neuron 1994, 12, 707–716.
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J 2008, 413, 201–215.
- Saxena, S.K.; Kaur, S. Regulation of epithelial ion channels by Rab GTPases. Biochem. Biophys. Res. Commun 2006, 351, 582–587.
- Kanamarlapudi, V. Centaurin-alpha1 and KIF13B kinesin motor protein interaction in ARF6 signalling. Biochem. Soc. Trans 2005, 33, 1279–1281.
- Saito, K.; Tautz, L.; Mustelin, T. The lipid-binding SEC14 domain. Biochim. Biophys. Acta 2007, 1771, 719–726.
- Stenmark, H.; Gillooly, D.J. Intracellular trafficking and turnover of phosphatidylinositol 3-phosphate. Semin. Cell Dev. Biol 2001, 12, 193–199.
- Shisheva, A. Phosphoinositides in insulin action on GLUT4 dynamics: Not just PtdIns(3,4,5)P3. Am. J. Physiol. Endocrinol. Metab 2008, 295, E536–E544.
