2. RUN Domain Binds Several Signaling Molecules
The RUN domains, named after RPIP8, UNC-14, and NESCA proteins [
11], might function as effectors of the small GTPase superfamily [
12,
13]. Because many RUN domain-containing proteins are involved in the signaling of small GTPases, including members of the Rap and Rab family, the RUN domain has been suggested to be involved in membrane trafficking [
13]. The RUN domain encloses hydrophobic amino acids in the conserved positions, which is a conserved protein motif that consists of approximately 200 amino acids with binding activity to small GTP-binding proteins [
11]. The sequence analysis has predicted that the RUN domain is composed of several conserved blocks, which constitute the core of a globular structure. The overall crystal structure of the RUN domain adopts a single globular fold consisting of eight alpha-helices [
14]. However, it is not the only function of the RUN domain to bind a GTPase. The RUN domain binds to some molecules, including motor proteins, and the RUN domain might be responsible for an interaction with a filamentous network [
13]. NESCA, a signaling adapter protein in the NGF-mediated signaling pathway, contains a RUN domain at the
N-terminus [
12]. The RUN domain of NESCA comprises nine helices, resembling the other RUN domain-containing proteins [
15]. Mutational analyses have demonstrated that the RUN domain is an important structural determinant for the nuclear translocation of NESCA and that the nuclear redistribution of NESCA is essential to its neurite outgrowth-promoting properties [
12]. However, the RUN domain of NESCA has different surface electrostatic distributions at the putative GTPase-interacting interface compared to the other RUN domains [
15]. The RUN domain of NESCA can bind H-Ras, a downstream signaling molecule of TrkA, as well as TrkA itself, suggesting that the NESCA participates in the NGF-TrkA signaling pathway [
15].
The RUN domain-containing proteins have been shown to promote endosomal fusion and are important for vesicular transport. In addition, the RUN domains appear to be required for localization to detergent-insoluble endosomal microdomains [
12,
13]. The physical interaction between RUN proteins and filamentous materials has been confirmed by several biochemical experiments using wild type and mutant proteins [
13]. The association among small GTPases, RUN proteins, and motor proteins might reflect a novel function for these proteins in the transport of vesicular cargoes in cells. It has been reported that FYCO1 functions as an adapter linking autophagosomes to microtubule molecular motors and the Rab7, which is implicated in the phagosomal transport and fusion [
16]. Kinesin-1 is a heterotetramer composed of kinesin heavy chain and kinesin light chain. UNC-14, a RUN domain protein binds to the kinesin-1 and regulates synaptic vesicle localization [
17]. UNC-14 is also predicted to play an important role in multiple Ras-like GTPase signaling pathways [
18]. Because RUN domains are often found in proteins involved in the regulation of Rab family small GTPases, the RUN domain has been suggested to be involved in the Rab-mediated membrane trafficking. It seems there is a common function underlying the mechanism for association of RUN domain to small GTPases and motor proteins.
3. Function of RUFY Family Proteins with the RUN Domain
The RUFY, designated as the RUN and FYVE domain-containing protein family, contains an amino-terminal RUN domain, and a carboxyl-terminal FYVE domain, which associate with phosphatidylinositol 3-phosphate in membranes of early endosomes [
19]. Actually, RUFY proteins are localized predominantly to the early endosomes. RUFY proteins are often tyrosine-phosphorylated and the mutant lacking the phosphorylation sites fails to go to the endosomes [
19]. It has been suggested Rab10, Rab11, Rab14, and RUFY proteins play an important role in the Glut4 trafficking in adipocytes and in skeletal muscle [
20]. Sequence and genome analysis has revealed that a RUFY family consists of 4 members of proteins (
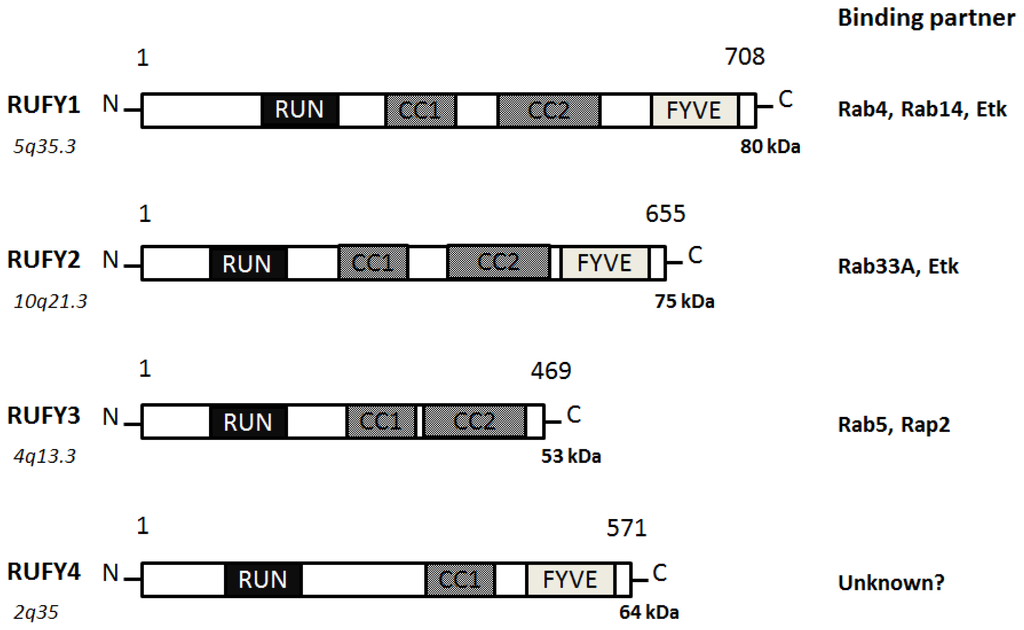
Figure 3).
Figure 3. Schematic diagram indicating the domain structures of the RUFY1, RUFY2, RUFY3, and RUFY4 proteins. The functionally important sites and their interaction proteins are shown. Genomic locations of each the genes and approximate molecular mass of each the proteins are also shown at the both ends. RUN = RPIP8, UNC-14, and NESCA proteins, FYVE = Fab-1, YGL023, Vps27, and EEA1 proteins, CC = Coiled Coil.
RUFY1, also known as RABIP4 or ZFYVE12, is a 708-amino acids protein that localizes to the cytoplasm and early endosome membrane [
19,
21]. RUFY1 has been identified as downstream effector of Etk protein kinase, which is highly expressed in testis, lung, brain and kidney. RUFY1 functions to bind PIP3-containing phospholipid vesicles and participates in early endosomal membrane trafficking [
19]. The downstream effects of PI3K signaling are mediated by proteins containing a PIP3-binding module indicated by the FYVE finger domain. Many FYVE domain-containing proteins are localized at the endosomes and play an important role in endocytosis [
22]. Through the SH3 and SH2 domains of Etk, the Etk interacts with RUFY1, then phosphorylates Tyr-281 and Tyr-292 of RUFY1, which is essential for the endosomal localization [
19]. The Etk plays an important role in the regulation of endocytosis as a downstream effector of PI3K. Two coiled coil domains also determine endosomal localization of RUFY1 [
23]. The PI3K inhibitor wortmannin blocks the endosomal localization of RUFY1 [
23]. Rab14 engages in a GTP-dependent interaction with RUFY1 [
24]. The active Rab14 regulates RUFY1 recruitment onto endosomal membranes, and Rab4 allows endosomal fusion [
24]. The Rab14 seems to be the primary determinant of RUFY1 recruitment to the endosomes, and the FYVE domain may assist RUFY1 targeting to PIP3-enriched early endosomes [
24]. Both Rab14 and RUFY1 are involved in Rab4-dependent recycling endosome, and enlargement of the early endosomes mediated by RUFY1 needs the interaction with Rab4 [
25]. The RUFY1 is present in the sorting endosomes, while Rab4 is present both on sorting and recycling endosomes [
26]. This would provide directional trafficking away from the recycling endosomes to the sorting endosomes. RUFY1 can also modify the kinetic parameters of Glut1 protein recycling [
26].
RUFY2, also known as RABIP4R or ZFYVE13, contains a RUN domain and a carboxy terminal FYVE zinc finger, separated by two coiled coil domains [
27]. Localizing to nucleus, RUFY2 is expressed in brain, lung and testis. RUFY2 as well as RUFY1 interacts with the Etk that is a tyrosine kinase involved in regulation of various cellular processes [
19]. The carboxyl domain of RUFY2 binds to a negative form of Rab33A [
28]. RUFY3, also known as RIPX or SINGAR1, is diffusely localized in hippocampal neurons and accumulated in the growth cones and axons [
29]. RUFY3 ensures the robustness of neuronal polarity by suppressing formation of surplus axons. RUFY3 also contains the RUN domain and seems to play important roles in multiple Ras-like GTPase signaling pathways. Rab5 engages in a GTP-dependent interaction with RUFY3. RUFY3 can bind to the active Rab5 and weakly associates to Rap2 [
30]. RUFY3 may function as a docking protein for distinct two small GTPases. It has been reported that oxidized LDL-containing immune complexes affect the gene expression of RUFY3 in human U937 monocytic cells [
31]. RUFY4 is a 571-amino acids protein that contains a RUN domain and a FYVE zinc finger domain [
32]. RUFY4 is also believed to be involved with zinc ion binding. As poorly characterized for RUFY2 and for RUFY4, little is known about the precise intracellular functions for these proteins.
4. Regulation of Cell Polarity and Membrane Trafficking
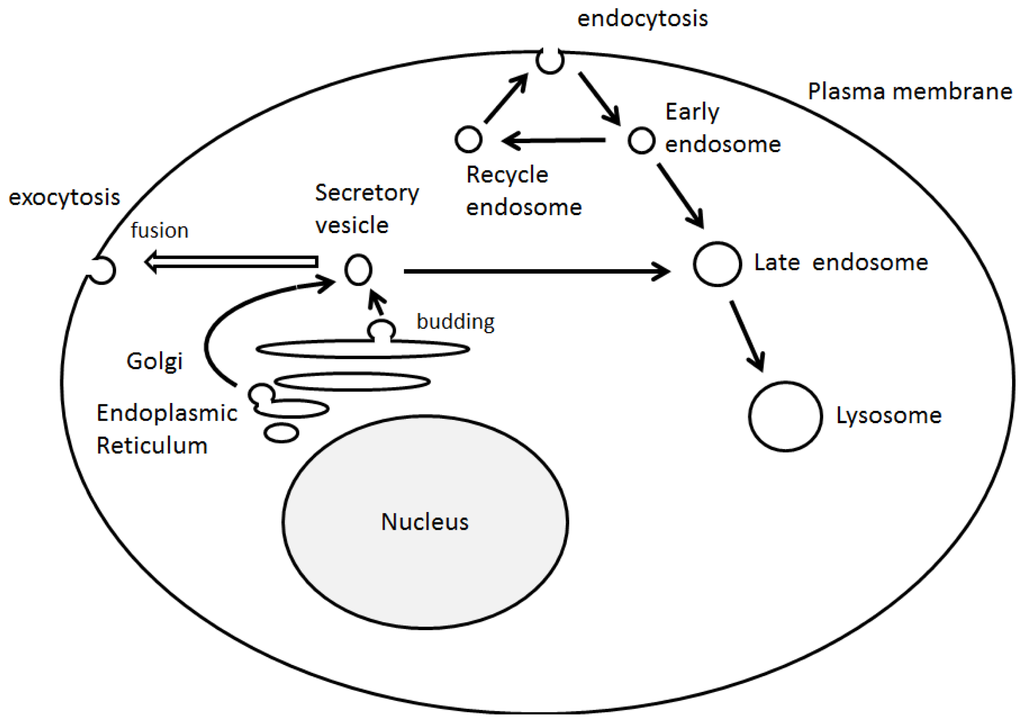
Cell polarity and vesicle sorting are significant processes that influence normal cell function including cell adhesion, migration, and neurotransmission. Endosomal membrane trafficking is a spatiotemporally regulated process that confirms suitable delivery of cargo via the pathway. Endosomes can bud inwardly from the membranes to form vesicles, which receive cargo from the cell surface via endocytosis and biosynthetic cargo from the late Golgi complex [
33]. The endocytic trafficking has been shown to be a critical component of many signaling pathways, which is indispensable for a wide range of developmental processes [
34]. In addition, the endosomal trafficking is regulated by sequential recruitment of a variety of cytosolic and membrane-bound proteins [
35]. Studies have elucidated many of the key components of the events that take place during the membrane trafficking. For example, small GTPases of Rab family, its effectors, Ca
2+ levels, and phosphoinositides may be all important. Previous studies have demonstrated that Ca
2+ influx triggers the final step in exocytotic membrane fusion events during neurotransmission [
36].
Similarly, phosphoinositides have been shown to be important for the recruitment of Rab family proteins and the binding factors [
37]. Plasma membrane channels are known to be regulated by the Rab proteins [
38] and phosphoinositides, which are molecules that determine the vesicular identity and direction of membrane trafficking. In particular, PIP3 is essential for the membrane trafficking of early endosome [
39]. The PIP3 has lots of effector proteins in mammalian cells, all of which contain PIP3-binding motifs such as FYVE and/or PH domains [
40]. Selective recruitment of these effectors by PIP3 may provide a mechanism by which the directionality for incoming vesicles and endosomes may be established. The PIP3 is thus critical for the maturation of endosomes, and for fusion events with intracellular organelles [
41]. Correspondingly, disruption of PIP3 synthesis by wortmannin, a PI3K inhibitor, affects the formation of internal vesicles and the maturation of endosomes [
42]. While phosphoinositides can recruit phosphoinositide-binding proteins to regulate the activity of GTPases, the GTPases can, in turn, control the activity of PIP-metabolizing enzymes.