The ketogenic diet has been shown to have a multifaceted effect on the prevention and treatment of cardiovascular diseases (CVD). Among other aspects, it has a beneficial effect on the blood lipid profile, even compared to other diets. It shows strong anti-inflammatory and cardioprotective potential, which is due, among other factors, to the anti-inflammatory properties of the state of ketosis, the elimination of simple sugars, the restriction of total carbohydrates and the supply of omega-3 fatty acids. In addition, ketone bodies provide “rescue fuel” for the diseased heart by affecting its metabolism. They also have a beneficial effect on the function of the vascular endothelium, including improving its function and inhibiting premature ageing. The ketogenic diet has a beneficial effect on blood pressure and other CVD risk factors through, among other aspects, weight loss. The evidence cited is often superior to that for standard diets, making it likely that the ketogenic diet shows advantages over other dietary models in the prevention and treatment of cardiovascular diseases.
- ketogenic diet
- cardiovascular disease
- prevention
- treatment
- KD
- CVD
- heart
1. Introduction
2. The Ketogenic Diet and Blood Lipid Profile
2.1. Lipid Profile and Cardiovascular Diseases
2.2. The Effect of the Ketogenic Diet on the Blood Lipid Profile
3. Anti-Inflammatory Potential of the Ketogenic Diet in Cardiovascular Diseases
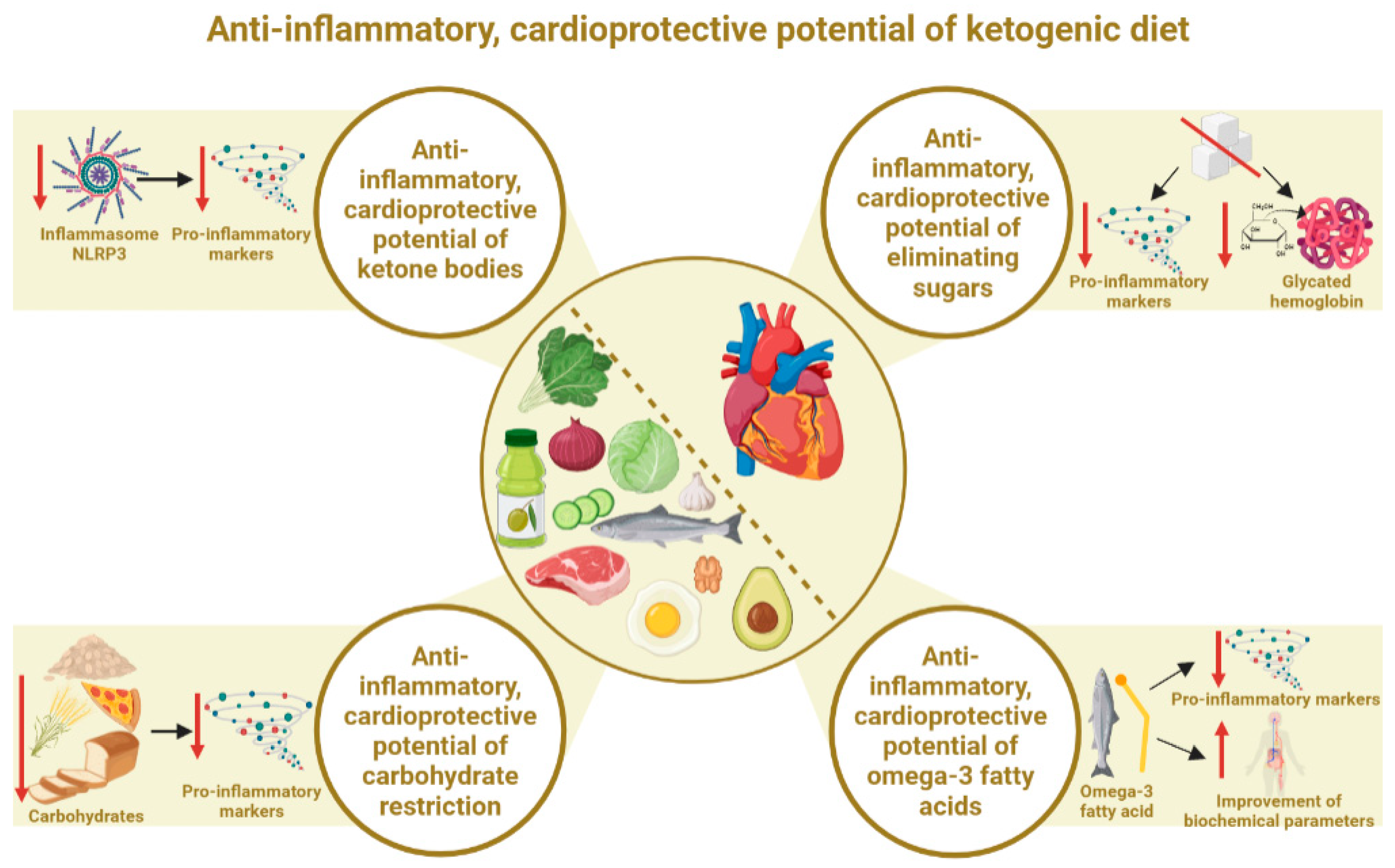
3.1. Anti-Inflammatory, Cardioprotective Potential of the State of Ketosis (Ketone Bodies)
3.2. Anti-Inflammatory, Cardioprotective Effects of Elimination of Simple Sugars
3.3. Anti-Inflammatory, Cardioprotective Effects of Total Carbohydrate Restriction
3.4. Anti-Inflammatory, Cardioprotective Effects of Omega-3 Fatty Acids
4. Ketone Bodies and Cardiac Energy Metabolism
5. The Ketogenic Diet and the Vascular Endothelium
6. The Ketogenic Diet and Blood Pressure
7. The Ketogenic Diet and Weight Loss as a Factor in CVD Prevention and Therapy
8. The Effect of the Ketogenic Diet among Patients with CVD and Healthy People
9. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/nu15153368
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639, Erratum in Circulation 2022, 146, e141.
- Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 4 March 2023).
- Available online: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm (accessed on 4 March 2023).
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. MentalStress and Cardiovascular Health—Part I. J. Clin. Med. 2022, 11, 3353.
- Cosentino, N.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603.
- Muzaffar, R.; Khan, M.A.; Mushtaq, M.H.; Nasir, M.; Khan, A.; Haq, I.U.; Muhammad, J. Hyperhomocysteinemia as an Independent Risk Factor for Coronary Heart Disease. Comparison with Conventional Risk Factors. Braz. J. Biol. 2021, 83, e249104.
- Khan, M.S.; Saeedullah, A.; Andrews, S.C.; Iqbal, K.; Qadir, S.A.; Shahzad, B.; Ahmed, Z.; Shahzad, M. Adolescent Afghan Refugees Display a High Prevalence of Hyperhomocysteinemia and Associated Micronutrients Deficiencies Indicating an Enhanced Risk of Cardiovascular Disease in Later Life. Nutrients 2022, 14, 1751.
- Xiao, K.; Chen, Y.; Xiao, L.; Sun, H.; He, Z.; Huang, G.; Chen, L.; Xv, L.; Peng, L.; Li, J.; et al. The relationship between hyperhomocysteinemia and total coronary artery occlusion: A cross-sectional study from Southwest China. Coron. Artery Dis. 2023, 34, 138–145.
- Tyrovola, D.; Soulaidopoulos, S.; Tsioufis, C.; Lazaros, G. The Role of Nutrition in Cardiovascular Disease: Current Concepts and Trends. Nutrients 2023, 15, 1064.
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian Dietary Patterns and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61.
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, 596–646.
- Mitrou, P.N.; Kipnis, V.; Thiébaut, A.C.; Reedy, J.; Subar, A.F.; Wirfält, E.; Flood, A.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F.; et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIH–AARP diet and health study. Arch. Intern. Med. 2007, 167, 2461–2468.
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100.
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338.
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984.
- Wilson, J.; Lowery, R. The Ketogenic Bible; Victory Belt Publishing Inc.: Las Vegas, NV, USA, 2017; ISBN 13:978-1-628601-04-6.
- Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Joseph, J.J. American Heart Association Council on Lifestyle and Cardiometabolic Health. Popular Dietary Patterns: Alignment with American Heart Association 2021 Dietary Guidance: A Scientific Statement from the American Heart Association. Circulation 2023, 147, 1715–1730.
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363.
- Jung, E.; Kong, S.Y.; Ro, Y.S.; Ryu, H.H.; Shin, S.D. Serum Cholesterol Levels and Risk of Cardiovascular Death: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2022, 19, 8272.
- Dong, J.; Yang, S.; Zhuang, Q.; Sun, J.; Wei, P.; Zhao, X.; Chen, Y.; Chen, X.; Li, M.; Wei, L.; et al. The Associations of Lipid Profiles With Cardiovascular Diseases and Death in a 10-Year Prospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 745539.
- Yi, S.W.; Yi, J.J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596.
- BHF-HEART STATS and WHO-MORTALITY (Adapted). Total Cholesterol Levels vs Mortality Data from 164 Countries, 2005. Available online: https://renegadewellness.files.wordpress.com/2011/02/cholesterol-mortality-chart.pdf (accessed on 23 July 2023).
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330.
- Peng, K.M.; Li, X.; Wang, Z.; Li, M.M.; Yang, Y. Association of low-density lipoprotein cholesterol levels with the risk of mortality and cardiovascular events: A meta-analysis of cohort studies with 1,232,694 participants. Medicine 2022, 101, e32003.
- Bhargava, S.; de la Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and lipoproteins in cardiovascular diseases: A classification. Trends Endocrinol. Metab. 2022, 33, 409–423.
- Kim, Y.G.; Jeong, J.H.; Han, K.D.; Roh, S.Y.; Min, K.; Lee, H.S.; Choi, Y.Y.; Shim, J.; Choi, J.I.; Kim, Y.H. Association between low-density lipoprotein cholesterol and sudden cardiac arrest in people with diabetes mellitus. Cardiovasc. Diabetol. 2023, 22, 36.
- Rong, S.; Li, B.; Chen, L.; Sun, Y.; Du, Y.; Liu, B.; Robinson, J.G.; Bao, W. Association of Low-Density Lipoprotein Cholesterol Levels with More than 20-Year Risk of Cardiovascular and All-Cause Mortality in the General Population. J. Am. Heart Assoc. 2022, 11, e023690.
- Nicholls, S.J.; Nelson, A.J. HDL and cardiovascular disease. Pathology 2019, 51, 142–147.
- Trimarco, V.; Izzo, R.; Morisco, C.; Mone, P.; Virginia Manzi, M.; Falco, A.; Pacella, D.; Gallo, P.; Lembo, M.; Santulli, G.; et al. High HDL (High-Density Lipoprotein) Cholesterol Increases Cardiovascular Risk in Hypertensive Patients. Hypertension 2022, 79, 2355–2363.
- Cho, Y.K.; Jung, C.H. HDL-C and Cardiovascular Risk: You Don’t Need to Worry about Extremely High HDL-C Levels. J. Lipid Atheroscler. 2021, 10, 57–61.
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch Cardiovasc. Dis. 2021, 114, 132–139.
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34.
- Ravnskov, U.; de Lorgeril, M.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Mascitelli, L.; et al. LDL-C does not cause cardiovascular disease: A comprehensive review of the current literature. Expert Rev. Clin. Pharmacol. 2018, 11, 959–970.
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401.
- Ravnskov, U.; de Lorgeril, M.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjöld, B.; Harcombe, Z.; Kendrick, M.; Langsjoen, P.H.; McCully, K.S.; et al. The LDL paradox: Higher LDL-cholesterol is associated with greater longevity. Ann. Epidemiol. Public Health 2020, 3, 1040–1047.
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879.
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599.
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414.
- Mercurio, V.; Lobasso, A.; Barbieri, L.; Parrella, P.; Ciervo, D.; Liccardo, B.; Bonaduce, D.; Tocchetti, C.G.; De Paulis, A.; Rossi, F.W. Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2019, 20, 2154.
- Varricchi, G.; Loffredo, S.; Borriello, F.; Pecoraro, A.; Rivellese, F.; Genovese, A.; Spadaro, G.; Marone, G. Superantigenic Activation of Human Cardiac Mast Cells. Int. J. Mol. Sci. 2019, 20, 1828.
- Brigant, B.; Metzinger-Le Meuth, V.; Rochette, J.; Metzinger, L. TRIMming down to TRIM37: Relevance to Inflammation, Cardiovascular Disorders, and Cancer in MULIBREY Nanism. Int. J. Mol. Sci. 2018, 20, 67.
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131.
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906.
- Sklerov, M.; Dayan, E.; Browner, N. Functional neuroimaging of the central autonomic network: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 555–566.
- Kraynak, T.E.; Marsland, A.L.; Gianaros, P.J. Neural Mechanisms Linking Emotion with Cardiovascular Disease. Curr. Cardiol. Rep. 2018, 20, 128.
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater amygdala activity and dorsomedial prefrontal–amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015, 43, 46–53.
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859.
- Shah, S.M.; Meadows, J.L.; Burg, M.M.; Pfau, S.; Soufer, R. Effects of Psychological Stress on Vascular Physiology: Beyond the Current Imaging Signal. Curr. Cardiol. Rep. 2020, 22, 156.
- Tawakol, A.; Ishai, A.; Takx, R.A.P.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.E.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845.
- Pondel, N.; Liśkiewicz, D.; Liśkiewicz, A. Dieta ketogeniczna–mechanizm działania i perspektywy zastosowania w terapii: Dane z badań klinicznych. Postępy Biochem. 2020, 66, 270–286.
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003.
- Hwang, C.Y.; Choe, W.; Yoon, K.-S.; Ha, J.; Kim, S.S.; Yeo, E.-J.; Kang, I. Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer. Nutrients 2022, 14, 4932.
- Dyńka, D.; Kowalcze, K.; Ambrozkiewicz, F.; Paziewska, A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients 2023, 15, 500.
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M.F.; Goel, A.; Qin, H.; et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct. Target. Ther. 2021, 6, 154.
- Field, R.; Pourkazemi, F.; Rooney, K. Effects of a Low-Carbohydrate Ketogenic Diet on Reported Pain, Blood Biomarkers and Quality of Life in Patients with Chronic Pain: A Pilot Randomized Clinical Trial. Pain Med. 2022, 23, 326–338.
- Alkhorayef, N.; Almutery, F.T.; Rasheed, Z.; Althwab, S.A.; Aljohani, A.S.M.; Alhawday, Y.A.N.; Salem, T.; Alharbi, A.M.; Wahaq, A.A.A.B.; Alharbi, F.S.; et al. Regulatory effects of ketogenic diet on the inflammatory response in obese Saudi women. J. Taibah Univ. Med. Sci. 2023, 18, 1101–1107.
- Mohammadifard, N.; Haghighatdoost, F.; Rahimlou, M.; Rodrigues, A.P.S.; Gaskarei, M.K.; Okhovat, P.; de Oliveira, C.; Silveira, E.A.; Sarrafzadegan, N. The Effect of Ketogenic Diet on Shared Risk Factors of Cardiovascular Disease and Cancer. Nutrients 2022, 14, 3499.
- Mezzaroma, E.; Toldo, S.; Farkas, D.; Seropian, I.M.; Van Tassell, B.W.; Salloum, F.N.; Kannan, H.R.; Menna, A.C.; Voelkel, N.F.; Abbate, A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. USA 2011, 108, 19725–19730.
- Tong, Y.; Wang, Z.; Cai, L.; Lin, L.; Liu, J.; Cheng, J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4293206.
- Zheng, Y.; Xu, L.; Dong, N.; Li, F. NLRP3 inflammasome: The rising star in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 927061.
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Lowcarbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284.
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481.
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606.
- O’Connor, L.; Imamura, F.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin. Nutr. 2018, 37, 1313–1322.
- McGandy, R.B.; Hegsted, D.M.; Stare, F.J. Dietary fats, carbohydratesand atherosclerotic vascular disease. N. Engl. J. Med. 1967, 277, 186–192.
- Carbone, S.; Billingsley, H.E.; Lavie, C.J. The Effects of Dietary Sugars on Cardiovascular Disease and Cardiovascular Disease-Related Mortality: Finding the Sweet Spot. Mayo Clin. Proc. 2019, 94, 2375–2377.
- Howard, B.V.; Van Horn, L.; Hsia, J.; Manson, J.E.; Stefanick, M.L.; Wassertheil-Smoller, S.; Kuller, L.H.; LaCroix, A.Z.; Langer, L.D.; Lasser, N.L.; et al. Low-fat dietary patternand risk of cardiovascular disease: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006, 295, 655–666.
- Billingsley, H.E.; Carbone, S.; Lavie, C.J. Dietary fats and chronicnoncommunicable diseases. Nutrients 2018, 10, 1385.
- Huang, C.; Huang, J.; Tian, Y.; Yang, X.; Gu, D. Sugar sweetened beverages consumption and risk of coronary heart disease: A metaanalysis of prospective studies. Atherosclerosis 2014, 234, 11–16.
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats comparedwith unsaturated fats and sources of carbohydrates in relationto risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548.
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortalityamong US adults. JAMA Intern. Med. 2014, 174, 516–524.
- Available online: https://www.health.harvard.edu/heart-health/the-sweet-danger-of-sugar (accessed on 23 July 2023).
- Kelly, R.K.; Tong, T.Y.N.; Watling, C.Z.; Reynolds, A.; Piernas, C.; Schmidt, J.A.; Papier, K.; Carter, J.L.; Key, T.J.; Perez-Cornago, A. Associations between types and sources of dietary carbohydrates and cardiovascular disease risk: A prospective cohort study of UK Biobank participants. BMC Med. 2023, 21, 34.
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155.
- Cheng, H.; Zhou, J.; Sun, Y.; Zhan, Q.; Zhang, D. High fructose diet: A risk factor for immune system dysregulation. Hum. Immunol. 2022, 83, 538–546.
- Lubawy, M.; Formanowicz, D. High-Fructose Diet–Induced Hyperuricemia Accompanying Metabolic Syndrome–Mechanisms and Dietary Therapy Proposals. Int. J. Environ. Res. Public Health 2023, 20, 3596.
- Rawal, G.; Yadav, S.; Kumar, R.; Singh, A. Glycosylated hemoglobin (HbA1C): A brief overview for clinicians. IP Indian J. Immunol. Respir. Med. 2016, 1, 33–36.
- Goto, A.; Noda, M.; Matsushita, Y.; Goto, M.; Kato, M.; Isogawa, A.; Takahashi, Y.; Kurotani, K.; Oba, S.; Nanri, A.; et al. JPHC Study Group. Hemoglobin a1c levels and the risk of cardiovascular disease in people without known diabetes: A population-based cohort study in Japan. Medicine 2015, 94, e785.
- Sinning, C.; Makarova, N.; Völzke, H.; Schnabel, R.B.; Ojeda, F.; Dörr, M.; Felix, S.B.; Koenig, W.; Peters, A.; Rathmann, W.; et al. Association of glycated hemoglobin A1c levels with cardiovascular outcomes in the general population: Results from the BiomarCaRE (Biomarker for Cardiovascular Risk Assessment in Europe) consortium. Cardiovasc. Diabetol. 2021, 20, 223.
- Prasad, K. Does HbA1cc Play a Role in the Development of Cardiovascular Diseases? Curr. Pharm. Des. 2018, 24, 2876–2882.
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Bidar, S.S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56.
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279.
- Liu, Y.-X.; Yu, J.-H.; Sun, J.-H.; Ma, W.-Q.; Wang, J.-J.; Sun, G.-J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725.
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146.
- Fatahi, S.; Sohouli, M.H.; da Silva Magalhães, E.I.; da Cruz Silveira, V.N.; Zanghelini, F.; Rahmani, P.; Kord-Varkaneh, H.; Sharifi-Zahabi, E.; Shidfar, F. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on cardiovascular risk factors: Pairwise and network meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 11–21.
- Yang, B.; Tseng, P.T.; Hu, X.; Zeng, B.Y.; Chang, J.P.; Liu, Y.; Chu, W.J.; Zhang, S.S.; Zhou, Z.L.; Chu, C.S.; et al. Comparative efficacy of omega-3 polyunsaturated fatty acids on major cardiovascular events: A network meta-analysis of randomized controlled trials. Prog. Lipid Res. 2022, 88, 101196, Erratum in Prog. Lipid Res. 2022, 101206.
- Yokoyama, Y.; Kuno, T.; Morita, S.X.; Slipczuk, L.; Takagi, H.; Briasoulis, A.; Latib, A.; Bangalore, S.; Heffron, S.P. Eicosapentaenoic Acid for Cardiovascular Events Reduction- Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Cardiol. 2022, 80, 416–422.
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807.
- Stoll, S.; Leimena, C.; Qiu, H. Mitochondria and Heart Disease; InTech: London, UK, 2018.
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutictarget in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. Available online: https://www.researchgate.net/publication/327299198_Mitochondria_and_Heart_Disease (accessed on 24 April 2023).
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368.
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595.
- Abdul Kadir, A.; Clarke, K.; Evans, R.D. Cardiac ketone body metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165739.
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669.
- Sack, M.N.; Rader, T.A.; Park, S.; Bastin, J.; McCune, S.A.; Kelly, D.P. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 1996, 94, 2837–2842.
- Carley, A.N.; Taegtmeyer, H.; Lewandowski, E.D. Matrix revisited: Mechanisms linking energy substrate metabolism to the function of the heart. Circ. Res. 2014, 114, 717–729.
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, e124079.
- Bedi, K.C.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016, 133, 706–716.
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705.
- Lommi, M.D.J. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996, 28, 665–672.
- Voros, G.; Ector, J.; Garweg, C.; Droogne, W.; Van Cleemput, J.; Peersman, N.; Vermeersch, P.; Janssens, S. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ. Heart Fail. 2018, 11, e004953.
- Gormsen, L.C.; Svart, M.; Thomsen, H.H.; Sondergaard, E.; Vendelbo, M.H.; Chris-tensen, N.; Tolbod, L.P.; Harms, H.J.; Nielsen, R.; Wiggers, H.; et al. Ketone Body Infusion with 3-Hydroxybutyrate Reduces Myocardial Glucose Uptake and Increases Blood Flow in Humans: A Positron Emission Tomography Study. J. Am. Heart Assoc. 2017, 6, e005066.
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE 2018, 13, e0190556.
- Lauritsen, K.M.; Søndergaard, E.; Luong, T.V.; Møller, N.; Gormsen, L.C. Acute Hyperketonemia Does Not Affect Glucose or Palmitate Uptake in Abdominal Organs or Skeletal Muscle. J. Clin. Endocrinol. Metab. 2020, 105, 1785–1790.
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113.
- Nappi, F.; Fiore, A.; Masiglat, J.; Cavuoti, T.; Romandini, M.; Nappi, P.; Avtaar Singh, S.S.; Couetil, J.-P. Endothelium-Derived Relaxing Factors and Endothelial Function: A Systematic Review. Biomedicines 2022, 10, 2884.
- Weis, E.M.; Puchalska, P.; Nelson, A.B.; Taylor, J.; Moll, I.; Hasan, S.S.; Dewenter, M.; Hagenmüller, M.; Fleming, T.; Poschet, G.; et al. Ketone body oxidation increases cardiac endothelial cell proliferation. EMBO Mol. Med. 2022, 14, e14753.
- Devaraj, S.; Cheung, A.T.; Jialal, I.; Griffen, S.C.; Nguyen, D.; Glaser, N.; Aoki, T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role inmicrovascular complications. Diabetes 2007, 56, 2790–2796.
- White, N.H. Diabetic ketoacidosis in children. Endocrinol. Metab. Clin. North Am. 2000, 29, 657–682.
- Bialo, S.R.; Agrawal, S.; Boney, C.M.; Quintos, J.B. Rare complications of pediatric diabetic ketoacidosis. World J. Diabetes 2015, 6, 167–174.
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141.
- DeFronzo, R.A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia 1981, 21, 165–171.
- Brands, M.W. Role of Insulin-Mediated Antinatriuresis in Sodium Homeostasis and Hypertension. Hypertension 2018, 72, 1255–1262.
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490.
- Harvey, C.J.D.C.; Schofield, G.M.; Williden, M. The use of nutritional supplements to induce ketosis and reduce symptomsassociated with keto-induction: A narrative review. PeerJ 2018, 6, e4488.
- Zupec-Kania, B.; Zupanc, M.L. Long-term management of the ketogenic diet: Seizure monitoring, nutrition, and supplementation. Epilepsia 2008, 49 (Suppl. S8), 23–26.
- Cordain, L. Nutritional Deficiencies of Ketogenic Diets. 2018. Available online: https://www.researchgate.net/publication/332098774_Nutritional_Deficiencies_of_Ketogenic_Diets?channel=doi&linkId=5c9f99e2a6fdccd46045868c&showFulltext=true (accessed on 23 July 2023). License CC BY-NC-ND 4.0.
- Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients 2019, 11, 1362.
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958.
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986.
- Polito, R.; Messina, G.; Valenzano, A.; Scarinci, A.; Villano, I.; Monda, M.; Cibelli, G.; Porro, C.; Pisanelli, D.; Monda, V.; et al. The Role of Very Low Calorie Ketogenic Diet in Sympathetic Activation through Cortisol Secretion in Male Obese Population. J. Clin. Med. 2021, 10, 4230.
- Polito, R.; Valenzano, A.; Monda, V.; Cibelli, G.; Monda, M.; Messina, G.; Villano, I.; Messina, A. Heart Rate Variability and Sympathetic Activity Is Modulated by Very Low-Calorie Ketogenic Diet. Int. J. Environ. Res. Public Health 2022, 19, 2253.
- Barrea, L.; Verde, L.; Camajani, E.; Šojat, A.S.; Marina, L.; Savastano, S.; Colao, A.; Caprio, M.; Muscogiuri, G. Effects of very low-calorie ketogenic diet on hypothalamic–pituitary–adrenal axis and renin–angiotensin–aldosterone system. J. Endocrinol. Investig. 2023, 46, 1509–1520.
- Belany, P.; Kackley, M.L.; Zhao, S.; Kluwe, B.; Buga, A.; Crabtree, C.D.; Nedungadi, D.; Kline, D.; Brock, G.; Simonetti, O.P.; et al. Effects of Hypocaloric Low-Fat, Ketogenic, and Ketogenic and Ketone Supplement Diets on Aldosterone and Renin. J. Clin. Endocrinol. Metab. 2023, 108, 1727–1739.
- Di Raimondo, D.; Buscemi, S.; Musiari, G.; Rizzo, G.; Pirera, E.; Corleo, D.; Pinto, A.; Tuttolomondo, A. Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients 2021, 13, 2567.
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Tas k Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309.
- Di Raimondo, D.; Musiari, G.; Miceli, G.; Arnao, V.; Pinto, A. Preventive and Therapeutic Role of Muscle Contraction against Chronic Diseases. Curr. Pharm. Des. 2016, 22, 4686–4699.
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts. 2021, 14, 222–245.
- Markovikj, G.; Knights, V.; Kljusurić, J.G. Ketogenic Diet Applied in Weight Reduction of Overweight and Obese Individuals with Progress Prediction by Use of the Modified Wishnofsky Equation. Nutrients 2023, 15, 927.
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356.
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010.
- Bueno, N.; De Melo, I.; De Oliveira, S.; Da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187.
- Choi, Y.J.; Jeon, S.M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005.
- Burén, J.; Ericsson, M.; Damasceno, N.R.T.; Sjödin, A. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814.
- Michalczyk, M.M.; Klonek, G.; Maszczyk, A.; Zajac, A. The Effects of a Low Calorie Ketogenic Diet on Glycaemic Control Variables in Hyperinsulinemic Overweight/Obese Females. Nutrients 2020, 12, 1854.
