1. Introduction
Neurodegenerative diseases are interlaced with the disorders of multicellular function and/or interaction in the central nervous system (CNS) [
1]. “Neurodegeneration” represents a condition where neuronal cells gradually lose their potential functions, and eventually perish. Alzheimer’s disease (AD) is the most common neurodegenerative disease. Neurodegenerative diseases have a huge impact on patients themselves, adding to the social economic burden [
2], which is regrettably expected to increase worldwide [
3]. Now, progress in resolving the mechanisms behind several neurodegenerative diseases has been considerably recognized. For example, epigenetic modifications and genetic risk factors may be implicated in their appearance, and their mixture could predict or support the identification of individuals at significant risk [
4]. In general, neurodegenerative diseases could result in motor dysfunctions and behavioral manifestations such as ataxia, and/or dementia [
5]. Clarification of pathogenic mechanisms and new targeted drugs have been urgently needed.
The most important advances in RNA-modification-mediated regulation of gene expression might be an evolving field of epitranscriptomics. The epitranscriptome may contain all the biochemical modifications of the RNA within cells, which is dynamically regulated by specific enzymatic reactions. These RNA modifications could regulate a variety of physiological RNA functions. More than hundred chemical alterations of RNAs have been found in all types of RNAs [
6]. The most notable RNA methylation may account for more than 60% of all RNA alterations. Among them, the N6-methyladenosine (m6A) might be a noticeable post-transcriptional RNA modification in eukaryotes, which could regulate the expression of various genes [
7,
8]. It is noteworthy that the m6A is highly enriched in adult brain tissue [
9], which might indicate that it plays a critical role in neurogenesis, neurodevelopment, and/or neurodegenerative disorders [
10]. In addition, the profusion of m6A in the brain seems to gradually increase with age from newborns, and reaches a peak in later life [
11]. Within the structure of total RNAs, the m6A is mostly dispersed in the 3’ untranslated region (UTR), coding sequence of message RNA (mRNA), and/or regions nearby the stop codon [
12]. The roles of the m6A modification in the control of gene expression may be strictly connected to various normal and/or pathological routes containing DNA damage response, cellular differentiation, and/or the occurrence of neurodegenerative diseases. In addition to the regulation of the transcriptional mRNA, m6A modification could also regulate the transcription of a variety of non-coding RNAs (ncRNAs) including microRNAs (miRNAs), circular RNAs (circRNAs), and/or long non-coding RNAs (lncRNAs) [
13]. Therefore, m6A modification could take part in the regulation of various processes in physiological cellular activities including cell development, embryonic development and/or stress responses, as well as in a regulatory role of several diseases [
14,
15]. The motif of m6A during the development may diverge across tissues and/or developmental courses [
16], in which the m6A modification may be inclined to the coding sequence and/or 3′UTR [
16]. Remarkably, even a single modification could have a vast effect on the function of RNAs. Interestingly, ncRNAs such as lncRNAs could also regulate the expression of m6A-related proteins [
17].
Studies have suggested that m6A modification on mRNAs could influence the proliferation and/or differentiation of neural progenitor cells in a comprehensive understanding of RNA methylation-based diagnosis and/or therapies for neurodegenerative diseases [
18,
19]. In addition, many studies have also demonstrated the effects of m6A modification in the autophagy mechanisms [
20], suggesting that the m6A change might play a critical role in controlling the development of neurodegenerative diseases via the control of autophagy. For example, the m6A modification could instruct direct inhibitory effects on autophagy [
21]. Moreover, the m6A modification could influence the construction of autophagosomes to dysregulate autophagy [
22]. Occasionally, the m6A modification could even initiate and/or promote autophagy [
23]. Interestingly, the properties of the m6A modification for autophagy may be reliant on disease situation and/or stages. Accordingly, abnormal m6A methylation could modify biological processes and/or regulate various human disorders [
24]. Therefore, the roles of m6A modifying enzymes might be involved in the pathological process of neurodegenerative diseases, which might be of huge importance to the development of a novel tactic for specific therapy of neurodegenerative diseases. In this report, we will go over the main points of the latest studies describing the impact on m6A modification in neurodegenerative diseases via the regulation of autophagy, and discuss the role of the m6A modification–autophagy relationship for the promising treatment of neurodegenerative diseases (
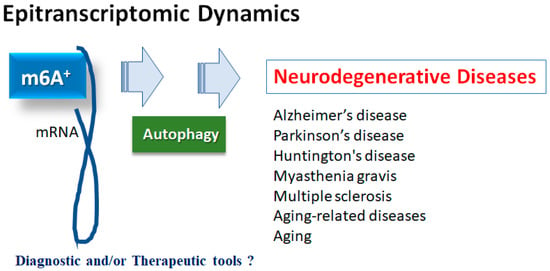
Figure 1).
Figure 1. Illustration of the relation of m6A RNAs methylation to various neurodegenerative diseases. Roles of m6A RNAs methylation with autophagy have been proposed in several neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, myasthenia gravis and multiple sclerosis, as well as in aging-related diseases and aging. Consequently, m6A RNAs methylation could be diagnostic and/or therapeutic tools for these diseases.
2. m6A Methylation of mRNAs in Neurodegenerative Diseases
2.1. Alzheimer’s Disease
The m6A abnormality is directly related to AD [
31], which may be one of the leading reasons of dementia and an increasing health problem worldwide. There is still no cure for AD. AD may be characterized by synaptic loss, hyper-phosphorylation of tau, extracellular plaques of amyloid-beta accumulation, and various levels of neuro-inflammation, as in neurodegenerative diseases [
32]. Several biological processes may be exaggerated by epitranscriptomic modifications, which could control the expression of specific genes via the metabolism of mRNAs. In response to modifications of certain mRNAs by m6A methylation, the nervous system could become dysfunctional, which might play an important role in the development of AD. In general, the N6-methyladenosine levels are high in the brain [
33]. Correspondingly, the expression of multiple m6A methylation regulators could change when AD occurs. In particular, m6A methylation levels are significantly elevated in the cerebral cortex and/or hippocampus of AD model mice, possibly due to the elevated expression of the m6A methyltransferase and/or the decreased expression of the m6A demethylase, which might be associated with the membrane synaptic growth [
31,
34]. It has been reported that the methyltransferase-like 3 (METTL3) may be downregulated at the hippocampus in human AD samples, and upregulation of METTL3 could promote autophagic p-Tau clearance and ameliorate AD both in vitro and in vivo [
35]. In addition, the m6A could control protein levels of key genes involved in AD-associated pathways [
33,
36].
2.2. Parkinson’s Disease
Parkinson’s disease (PD) might be the second most-frequent neurodegenerative disease after AD. It has been shown that multiple m6A methylated proteins are associated with PD. The m6A modifications could also regulate dopaminergic signaling. For example, the deletion of METTL14 in the substantia nigra could reduce the level of m6A mRNAs, and might impair the autonomic activity in mice [
37]. Overexpression of the fat mass and obesity-associated (FTO) gene and m6A inhibitor cycloleucine could reduce m6A levels in PD models [
38]. It has been shown that manganese exposure is a major environmental cause of PD. Remarkably, FTO overexpression could also diminish the manganese-induced cytotoxicity, and thereby could improve the symptoms of PD [
39]. Accordingly, it is suggested that some inhibitors of FTO might be utilized for the treatment of PD. For example, entacapone is a catechol-O-methyltransferase inhibitor approved as an adjunctive therapy in combination with levodopa for PD treatment which could promote the modification of the target gene forkhead box protein O1 (FOXO1)-m6A by decreasing FTO expression [
40].
2.3. Neurodegenerative Disorders and Aging
Huntington’s disease is an autosomal dominant neurodegenerative disorder, which is characterized by choreiform movements, cognitive deficits, and/or psychiatric symptoms [
41]. These impairments may be attributed to hippocampal dysfunction and/or corticostriatal dysfunction [
42]. Altered m6A RNA methylation as a unique hallmark of Huntington’s disease might contribute to hippocampal memory deficits in model mice of Huntington’s disease [
43]. Huntington’s disease is an autoimmune disease of the nervous system mainly caused by the autoantibodies against the acetylcholine receptor (AChR) in the postsynaptic membranes of the neuromuscular junction [
44]. It has been suggested that myasthenia gravis may be a complex multifactorial disease involving environmental, genetic, and/or immunological factors. Among them, the m6A regulators might play imperative roles in the pathogenesis of myasthenia gravis [
45]. Multiple sclerosis is a neuro-inflammatory disease that affects the brain, spinal cord, and optic nerves [
46]. Unfortunately, there is also no permanent cure for this neurological disorder. Optimal treatment regimens should be agreed upon for a good quality of life (QOL) [
47]. The m6A RNA modifications might be closely associated with the development of multiple sclerosis [
48]. It is noteworthy that cellular senescence is an important component of the aging process. It has been suggested that aging could definitely affect m6A RNAs methylation in the hippocampal regions [
49]. In addition, there are aging-related differences in the modification level of the epigenome m6A [
50]. Additionally, the reversibility of the m6A modification in aging may indicate its possibility for delaying aging.
3. ROS, Inflammation and m6A mRNAs
The m6A modification is the most dynamic and reversible epigenetic modification of eukaryotic mRNAs [
51], which could be regulated by methyltransferases and/or demethylases [
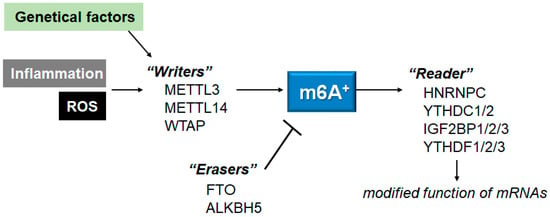
52]. Methyltransferases, also called “writers”, may be composed of METTL3, methyltransferase-like 14 (METTL14), and/or Wilms tumor 1-associated protein (WTAP), which could catalyze the methylation of N6-adenosine with the function of methyl connection [
53]. The METTL3 might be the catalytic subunit, while the METTL14 may be involved in the stability of the complex, as well as the RNA recruitment. The WTAP may also be required for the recruitment of mRNA. Among them, the METTL3 has been described as playing imperative roles in many pathological processes, predominantly in inflammatory and/or autoimmune responses [
53]. m6A-RNAs binding proteins, also called “readers”, may generally contain heterogeneous nuclear ribonucleoprotein C (HNRNPC), YTH domain-containing protein 1/2 (YTHDC1/2), insulin-like growth factor 2 mRNA-binding protein 1/2/3 (IGF2BP1/2/3), and/or YTH domain-containing family protein 1/2/3 (YTHDF1/2/3). Reader molecules might be prepared for distinguishing the m6A motif, thereby accomplishing the modification of function on the m6A-RNA [
54]. Additionally, the m6A modification could be detached by demethylases, also known as “erasers”, including alkB homolog 5 (ALKBH5) and/or FTO (
Figure 2). For example, the FTO could favorably demethylate the m6A located nearer to the mRNA cap.
Figure 2. Schematic representation of the players involved in the m6A RNAs methylation. The m6A modification is regulated by methyltransferases “writers” and demethylases “erasers”. The m6A-RNAs binding proteins are called “readers”. Example molecules are also shown for each player. Genetic factors and/or inflammation with ROS may affect the function of these players. The arrowhead means stimulation, and the hammerhead represents inhibition. Note that some critical pathways have been omitted, for clarity.
For example, the half-life of myosin heavy chain 3 (Myh3) mRNA could be significantly decreased after METTL3 deletion, in which Myh3 might have several potential m6A modification sites. Downregulation of METTL3 could reverse the lipopolysaccharide (LPS)-induced myocardial cell damage, largely by increasing Myh3 mRNA stability [
55]. In addition, the deletion of METTL3 could also increase the expression of myeloid differentiation factor 88 (MyD88), which may prevent the activation of NF-κB signaling in the LPS-treated cells [
56]. Similarly, the silencing of FTO could suppress the proliferation and/or invasion of cervical cancer cells via the m6A modification of the myelocytomatosis oncogene (Myc) and the zinc finger E-box-binding homeobox 1 (ZEB1) [
57]. The silencing of FTO could also inhibit the NLRP3-mediated IL-1β expression through the modification of the NF-κB signaling [
58], which may be related to the protection of cells.
Methylation put in by the “writers” could be reversed by “erasers” [
59], which might be controlled for healthy cellular homeostasis. Therefore, dysregulations of m6A might be linked to the perturbations of cell death and/or proliferation in diverse diseases [
60]. Accordingly, m6A modifications are potential novel diagnostic and/or therapeutic targets for several diseases, including neurodegenerative disease [
61]. As mentioned above, the m6A modification may be vigorously controlled by RNA methyltransferases (also known as “writers”), RNA demethylases (“erasers”) and RNA-binding proteins (“readers”) [
62]. Interestingly, the m6A level of RNAs may be significantly increased with the treatment of LPS [
63]. In addition, METTL3 expression might also be upregulated via the increased ROS production [
64]. For example, the ROS content might be elevated with the concomitant increase in m6A methylation in the liver after LPS treatment [
65]. Therefore, the m6A methylation of RNAs could be modulated under oxidative stresses [
65]. Furthermore, ROS could significantly increase the expression of YTHDF2, with the associated elevation of m6A methylation. Gathering this evidence has suggested that the alteration of the m6A modification may be a prevalent phenomenon under oxidative stress conditions. Therefore, immunological and/or inflammatory stresses could affect the m6A level of mRNAs [
66]. In general, ROS could regulate major epigenetic processes in various cells [
67,
68]. In particular, the change in ROS levels might contribute to the m6A RNA methylation [
69]. Consequently, the m6A methylation may be related to the increased levels of ROS content.
This entry is adapted from the peer-reviewed paper 10.3390/neurolint15030062