Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pathology
Venous thromboembolism (VTE), a common condition in Western countries, is a cardiovascular disorder that arises due to haemostatic irregularities, which lead to thrombus generation inside veins. Even with successful treatment, the resulting disease spectrum of complications considerably affects the patient’s quality of life, potentially leading to death. Cumulative data indicate that long non-coding RNAs (lncRNAs) may have a role in VTE pathogenesis.
- RNA
- long non-coding
- venous thromboembolism
1. Introduction
The coagulation system plays a leading role in VTE pathogenesis. Inclusively, treatment with anticoagulants constitutes the standard therapeutic approach to treat VTE events [132]. The most used anticoagulants are low molecular weight heparin (LMWH), unfractionated heparin (UFH) and direct oral anticoagulants (DOACs). LMWH and UFH bind to antithrombin III to inhibit the activity of several clotting factors, namely thrombin (or activated coagulation factor II (FIIa)) and activated coagulation factor X (FXa). On the other hand, DOACs act by inhibiting either thrombin or FXa, depending on the drug used [133].
The coagulation cascade involves a series of sequential proteolytic activation reactions, with a complex interplay between several proteins named coagulation factors that culminate in fibrin formation. There are three major coagulation pathways: extrinsic (also called the tissue factor (TF) coagulation pathway) and intrinsic (contact coagulation pathway), which converge on a common coagulation pathway [134]. Thromboplastin, or TF, is a transmembrane protein located constitutively on the surface of all cells except for endothelial and blood cells. When damage to blood vessels occurs, TF is released into the bloodstream, initiating the extrinsic coagulation pathway. By combining coagulation factor VII (FVII), the complex TF-FVII (extrinsic tenase complex) activates coagulation factor X (FX) [135]. FXa then participates in the common coagulation pathway. On a phospholipid surface, FXa forms a complex with activated coagulation factor V (FVa), known as prothrombinase complex, that converts prothrombin (coagulation factor II (FII)) into thrombin. Next, thrombin transforms fibrinogen (coagulation factor I (FI)) into fibrin (activated FI (FIa)) that deposits at the vascular injury site, forming a network together with platelets to create a blood clot [136]. Parallelly, the intrinsic coagulation pathway is initiated by factors released into the surrounding damaged tissue and circulating blood after injury, such as collagen and other negatively charged surfaces. Once activated, coagulation factor XII (FXII) activates coagulation factor XI (FXI), which then activates coagulation factor IX (FIX). In the presence of calcium ions (coagulation factor IV (FIV)), activated FIX (FIXa) forms a complex with coagulation factor VIII (FVIII) that generate FXa [137]. Both extrinsic and intrinsic pathways thus produce FXa, which then participates in the common coagulation pathway leading to thrombin generation and, ultimately, fibrin deposition (Figure 1A) [136]. To be noted, thrombin also activates thrombin activatable fibrinolysis inhibitor (TAFI), FV, FVIII, FXI and coagulation factor XIII (FXIII), creating a positive feedback loop that enhances clot formation. In addition, thrombin also promotes platelet adhesion by inactivating a disintegrin and metalloprotease with thrombospondin type 1 motif (ADAMTS13). On the other hand, this multifunction protein has anticoagulant activity by activating protein C, a natural anticoagulant that regulates the blood coagulation system through the inactivation of FVa and FVIIIa [138].
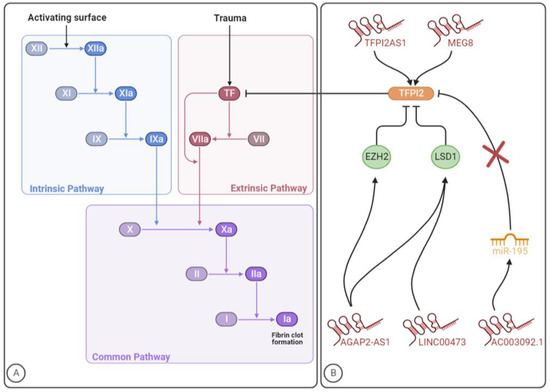
Figure 1. The involvement of lncRNAs in the clotting cascade. (A) Coagulation cascade. (B) LncRNAs that regulate TFPI2 expression/activity. The figure was created with BioRender.com (accessed on 10 July 2023).
Until now, few studies have been conducted to identify lncRNAs targeting the coagulation system, which were focused on the tissue factor pathway inhibitor 2 (TFPI2). As the name already indicates, TFPI is a serine protease inhibitor that blocks the activity of the extrinsic or TF coagulation pathway [139]. Specifically, under physiological conditions, this natural anticoagulant protein is known to damp coagulation by effectively inhibiting the extrinsic tenase complex and the prothrombinase complex [140,141]. Beyond coagulation disorders, deregulated levels of TFPI were shown in several diseases, such as cancer, diabetes mellitus and renal diseases [142,143,144,145,146,147,148,149,150]. Tissue factor pathway inhibitor 1 (TFPI1) and TFPI2 are two distinct proteins (encoded by different genes) with the role of maintaining the haemostatic balance [151]. Alternative splicing of TFPI1 leads to the synthesis of two primary isoforms, namely TFPIα and TFPIβ. The former is a soluble protein released into the bloodstream by endothelial cells and activated platelets, while the latter is a glycosylphosphatidyl inositol-anchored protein presented at the endothelium surface. Moreover, TFPIα inhibits both extrinsic tenase and prothrombinase, whereas TFPIβ blocks the extrinsic tenase complex more effectively, not being able to inhibit the prothrombinase complex [139]. As for TFPI2, it has inhibitory activity towards tenase complex, FXIa, plasmin and some matrix metalloproteinases [142,149]. Reduced synthesis of TFPI2 has been associated with angiogenesis, inflammation, atherosclerosis and tumour growth and metastasis [152].
A total of five studies from 2017 to 2022 were performed identifying five lncRNAs with implications in TFPI2 expression and/or activity (Table 1). The mechanisms through which these lncRNAs might impact TFPI2 and, consequently, blood coagulation, are summarized in Figure 1B. Given the role of the TF coagulation pathway -in VTE development, further investigation should be carried out to explore whether lncRNAs regulating TFPI2 expression might impact the disease pathogenesis and dissect the underlying mechanisms of action.
2. TFPI2AS1
TFPI2 antisense RNA 1 (TFPI2AS1) is an antisense lncRNA that is reported to positively regulate TFPI2 expression in NSCLC tissues, acting as a guide. Specifically, TFPI2AS1 is upregulated and inhibits tumour cell proliferation and metastasis by upregulating TFPI2 expression, although the underlying mechanisms are unclear [153]. Beyond cancer-induced hypercoagulability, dysregulation of TF-associated signalling pathways and their constituents play an important role in tumour progression, particularly by impacting angiogenesis and tumour invasion. While TF shows tumour-enhancing characteristics, its natural inhibitors, TFPI1 and TFPI2, are associated with tumour-suppressing properties [154].
3. Linc00473
Long intergenic ncRNA 00473 (linc00473) is a lincRNA that has been consistently linked to cancer cell proliferation, survival and metastasis [155,156]. Preeclampsia is a poorly comprehended pathological condition characterized by high blood pressure (hypertension) and proteinuria in the second half of pregnancy [157]. In a study aiming to explore the role of linc00473 in preeclampsia, this lncRNA was found to be downregulated in patients’ placenta, while in vitro, the lncRNA overexpression stimulated trophoblast proliferation. Acting as a molecular guide, Linc00473 was found to bind to lysine-specific demethylase 1 (LSD1), responsible for the demethylation of histone H3 lysine 4 dimethylation (H3K4me2) and histone H3 lysine 9 dimethylation (H3K9me2), thus affecting gene expression and inhibiting the expression of TFPI2 [158]. The role of TFPI2 in preeclampsia is, however, still unclear since both its increased and decreased levels have been reported in preeclamptic patients. According to the current evidence, TFPI2 levels might depend on the expression of glypican-3, a TFPI2-binding protein in placental tissue [159].
4. AC003092.1
Among glioblastoma patients, the lincRNA AC003092.1 was correlated with increased temozolomide (TMZ) resistance, higher risk of disease relapse and poor prognosis. Even though this focused on AC003092.1 as a potential therapeutic target for glioblastoma patients, the conducted investigation indicated that this lncRNA regulates TFPI2 expression through the miR-195/TFPI2 axis in glioblastoma. Specifically, by acting as an endogenous CeRNA and, consequently, as a decoy, AC003092.1 prevents miR-195 from targeting TFPI2, thereby increasing TFPI2 expression [160].
5. AGAP2-AS1
AGAP2 antisense RNA 1 (AGAP2-AS1) is a lincRNA that was first found overexpressed in human NSCLC, being also implicated in other malignant diseases such as colorectal cancer and melanoma [161,162,163]. By interacting with specific RNA-binding proteins, namely enhancers of zeste 2 polycomb repressive complex 2 subunit (EZH2) and LSD1, some lncRNAs can regulate cell phenotypes. The former is a subunit of polycomb repressive complex 2 (PRC2) with catalytic activity, which can suppress gene expression by enhancing histone H3 lysine 27 trimethylation (H3K27me3). As for LSD1, it can repress transcriptional activity through the enzymatic demethylation of histone H3 lysine 4 mono- and dimethylation (H3K4me1/2). In glioblastoma, AGAP2-AS1 acts as a guide and is suggested to inhibit TFPI2 expression through EZH2 and LSD1 binding [164].
6. MEG8
Long non-coding maternally expressed 8 (MEG8) was shown to be dysregulated in several disorders, such as lung, ovarian and colorectal cancer as well as gestational diabetes mellitus and diabetic nephropathy [165]. A study regarding ischemic heart disease revealed an induction of this gene in patients with this disease. By modulating the expression of TFPI2, which is a known angiogenesis inhibitor, MEG8 is suggested to regulate the angiogenic sprouting [166,167]. In concordance, experiments with HUVECs showed that TFPI2 was five times more expressed after MEG8 silencing. The negative regulation of this lncRNA reduces the inhibitory histone modification H3K27me3 at the TFPI2 promoter, therefore acting as a scaffold. Endothelial function is impaired by MEG8 silencing, suggesting a beneficial role of this lncRNA in preserving cell viability. Knowing that TFPI2 is an angiogenesis inhibitor and the role it plays in the coagulation cascade and extracellular matrix remodelling, the MEG8/TFPI2 axis could be a potential therapeutic target for VTE management [166].
Table 1. LncRNAs that regulate TFPI2 expression or activity.
| LncRNA | First Author (Country, Year) [Ref] | LncRNA Location 1 | Disease | Sample/Compartment or Study Model | LncRNA Expression in the Disease | LncRNA Targets |
LncRNA Role in TFPI2 Expression |
|---|---|---|---|---|---|---|---|
| TFPI2AS1 | Gao et al. (China, 2017) [153] | 7q31-q32 | NSCLC | NSCLC tissue and cells | ↑ | TFPI2 | ↑ |
| Linc00473 | Wu et al. (China, 2018) [158] | 6q27 | Preeclampsia | Placenta tissues and trophoblast cell lines | ↓ | LSD1/TFPI2 | ↓ |
| AC003092.1 | Xu et al. (China, 2018) [160] | 7q21.3 | Glioblastoma | Glioblastoma tissue and cells and mice | ↓ | MiR-195/TFPI2 | ↑ |
| AGAP2-AS1 | Luo et al. (China, 2019) [164] | 12q14.1 | Glioblastoma | Glioblastoma tissue and cells and mice | ↑ | EZH2 and LSD1/TFPI2 | ↓ |
| MEG8 | Kremer et al. (2022, The Netherlands) [166] | 14q32.31 | Ischemic heart disease | Left ventricular tissues and HUVECs | ↓ | TFPI2 | ↑ |
This entry is adapted from the peer-reviewed paper 10.3390/ijms241512103
This entry is offline, you can click here to edit this entry!
