Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of many cancer types, including head and neck cancers (HNC). When checkpoint and partner proteins bind, these send an “off” signal to T cells, which prevents the immune system from destroying tumor cells. Immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of many cancer types, including head and neck cancers (HNC).
- immune checkpoint inhibitor
- T-cells
1. Introduction
The immune system is a dynamic and equipped mechanism, an intricate system of “recognition” and “on-off” switches. Unfortunately, cancers utilize this system to enable growth and escape. The role of the immune system in tumor regulation is particularly evident in the immunocompromised. Iatrogenic solid organ transplant, diabetes, autoimmunity requiring immunosuppressive therapy, HIV and hemoproliferative malignant disease or disorders and aging, are all associated with an increased risk of developing head and neck cancer (HNC) and worse outcomes [1][2][3][4][5][6][7][8][9][10][11]. Proliferating tumors utilize many forms of immunosuppression to tip the balance of immunoediting toward tumor progression [12]. Identifying therapies capable of shifting this balance back toward immunosurveillance should play an integral role in reducing morbidity- and mortality-associated HNC.
HNC, the sixth most common group of malignancies worldwide, results in 680,000 new cases annually, with squamous cell carcinoma (SCC) being the most common [13][14][15]. The incidence of HNC is increasing due to a range of factors including smoking, alcohol, human papillomavirus (HPV) infection and extended life expectancy [16].
Despite the vital role played by traditional therapies for HNSCC, namely surgery, radiotherapy and chemotherapy, prognosis remains poor and survival remains correlated to stage, with a 5-year survival rate of 50–60% and more than 60% presenting in the advanced stage [17][18]. More than 50% of HNSCCs have tumor recurrence and metastasis in less than 3 years [19]. Targeting the epidermal growth factor receptor (EGFR) was hailed a paradigm shift in personalizing HNSCC treatment, with the monoclonal antibody cetuximab demonstrating promise [20][21]; however, this has since demonstrated limited efficacy [22].
Compared with traditional therapies, new immunotherapy agents, namely antibodies targeting the PD-1/PD-L1 system, so-called immune checkpoint inhibitors (ICI) provide improved efficacy and comparatively lower toxicity for patients with advanced HNSCC [23][24][25][26]. KEYNOTE-048 (NCT02358031), a randomized open-label phase 3 study comparing the humanized monoclonal antibody pembrolizumab (Keytruda) targeting PD-1 alone or in conjunction with chemotherapy (platinum and 5-fluorouracil) against cetuximab with chemotherapy, demonstrated overall survival improvement in both treatment arms over standard-of-care therapy in recurrent or metastatic HNSCC [27]. Pembrolizumab was subsequently approved as a first-line therapeutic drug for patients with metastatic, unresectable and recurrent HNSCC. Unfortunately, the objective response rate (ORR) of pembrolizumab (or nivolumab/Optivo) in HNSCC is only 15%, with short-term durability [28][29]. In addition, immune-related adverse events (irAEs) secondary to immunotherapy treatment were identified in over 50% of patients, impacting clinical outcomes [30], with adverse-event-associated mortality evident in 0.3–1.3% of patients [31]. Common irAEs include gastrointestinal, dermatologic and endocrine toxicities, more specifically dermatitis, rash, nausea/vomiting, fever, headache, myalgia, hypothyroidism and fatigue [32]. Rarely, irAEs can be severe, resulting in carditis, nephritis, hepatitis, pneumonitis, gastrointestinal perforation and severe hematological dysfunction [33]. irAEs in ICI therapy have been associated with benefits, namely improvements in PFS, OS and ORR [34][35][36][37]. Consequently, balancing immunotherapy de-escalation or commencement of immunosuppressive therapy against a sub-optimal oncological outcome can be difficult.
Predictive biomarkers may be the key to identifying patients at risk of irAEs. To date, circulating blood counts and ratios, autoantibodies and autoantigens, microRNAs, gastrointestinal microbiome, T-cell diversification and expansion and cytokines are all being investigated; however, they remain to be validated for clinical use [38].
Biological, etiological, phenotypic and clinical heterogeneities characterize HNSCC and challenge the development of personalized medicine. However, poor survival, significant morbidity and compromised quality of life emphasize the requirement for innovative therapy. Immunoediting is the process through which the immune system can promote and constrain tumor development [39].
2. Immune Checkpoint Inhibitor Targets and Therapies
A successful objective ICI response revitalizes the immune system to recognize and target cancer cells. The roles of known key immune checkpoints CTLA-4, PD-1 and LAG-3 are summarized in Figure 1.
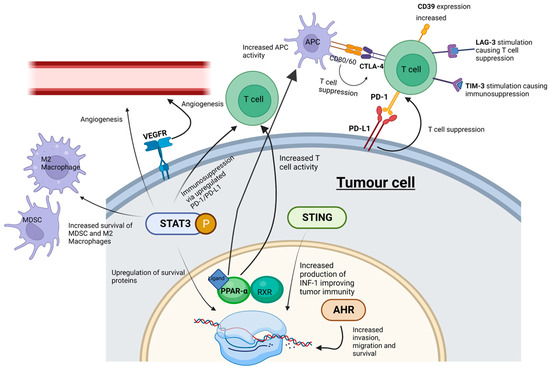
Figure 1. ICI and SMI actions within the tumor microenvironment. Whilst ICIs influence cell signaling at cell surface receptors, SMIs can interact with “upstream” intracellular signaling pathways-potentially playing a more effective role in abrogating tumor cell progression. MDSC, myeloid-derived suppressor cells; M2 macrophages, pro-tumorigenic macrophages; STING, stimulator of interferon genes; PPAR-α, peroxisome proliferator-activated receptor-α; AHR, aryl hydrocarbon receptor; STAT3, signal transducer and activator of transcription 3; P, phosphorylation of STAT3. Created with BioRender.com.
2.1. CTLA-4 and PD-1/PD-L1
CTLA-4 (cytotoxic T-lymphocyte associated protein 4, also known as cluster of differentiation 152, CD152) and programmed cell death protein 1 (PD-1) (and its ligands PD-L1 and PD-L2) are immune checkpoints targeted by humanized antibodies for the treatment of HNSCC. CTLA-4 is bound by ipilimumab (Yervoy), whereas PD-1 is targeted by pembrolizumab and nivolumab [32]. The antibodies atezolizumab (Tecentriq), durvalumab (Imfinzi) and avelumab (Bavencio) have also been approved as inhibitors of PD-L1 [33]. Both checkpoints regulate different stages of the immune response. CTLA-4 is considered the “leader” of the immune response and prevents the stimulation of autoreactive T-cells in the initial stage of naïve T-cell activation, whereas PD-1 is thought to regulate previously activated T-cells at the later stages of the immune response [32].
CTLA-4 is a homolog of CD28, but unlike CD28, CTLA-4 activation has an immunosuppressive effect opposite to the stimulatory effect of CD28 and the T-cell receptor (TCR) [40]. The binding of CD80/CD86 on antigen-presenting cells to CTLA-4 on T-cells in the tumor microenvironment suppresses the immune system, enabling tumor proliferation [41]. PD-1’s interaction with PD-L1 and PD-L2 has an immunosuppressive effect [41]. PD-L1 and PD-L2 are expressed by a range of tumors including HNSCC [42]. Critically, increased PD-1 levels serve as a biomarker for T cell exhaustion; this state of exhaustion is linked to T-cell dysfunction, which can facilitate tumor proliferation [43]. PD-L1′s interaction with PD-1 has an immunosuppressive effect, thus protecting cancer cells from lysis by activated T-cells [44].
Despite ICI therapy demonstrating survival advantage, comparatively few patients develop an effective response, the durability of which attenuates with acquired tumor resistance. Acquired resistance leads to tumor progression, and both arms of the immune system, innate and adaptive, can play a critical role in this change. Mechanisms of resistance to immunotherapy can be either intrinsic (tumor cell-mediated) or extrinsic (processes associated with T-cell activation) and shift the balance of immunomodulation towards tumor proliferation. Intrinsic resistance can include the downregulation of antigen-presenting machinery (APM) [45], the up-regulation of signaling pathways promoting T-cell exhaustion [46], the expression of multiple checkpoint inhibitors to mitigate T-cell activation [47], changes in tumor cell DNA repair, damage and genomic instability [48] and altered kinase signaling pathways [49]. Extrinsic resistance involves the complex interplay between tumor cells and the tumor microenvironment and its ability to regulate phenotypical characteristics of immune cells, especially TANs, TAMs, Tregs, MDSCs, T-cells, their associated regulatory cytokines and signaling pathways and a newly identified player, NETs [50][51][52][53][54][55][56].
Despite the clear improvements in overall survival due to immune checkpoint therapy, such treatments have limitations. For example, since CTLA-4 prevents the stimulation of autoreactive T-cells, inhibiting CTLA-4 can lead to grade 3 or 4 autoimmune-related adverse effects in 10–15% of patients [57]. Immune checkpoint immunotherapies are also associated with low response rates. For example, pembrolizumab has a response rate of only 15% in HNSCC [58].
To improve therapeutic failure and overcome immunotherapy resistance, significant energy is being invested in exploring biomarkers to predict clinical response and combinational therapies or changes in adjuvant delivery of immunotherapy to increase success rates. Biomarkers that have shown potential to determine improved clinical response in HNSCC include the tumor mutational burden, CCND1 amplification (CCND1 encodes cyclin D1, which regulates the retinoblastoma protein activity and cell-cycle progression), PD-1, IFN-γ, tumor-infiltrating lymphocytes (TILs) and cancer-associated fibroblasts (CAFs), CTLA-4, exosomes, CXCL, MTAP and SFR4/CPXM1/COL5A1 molecules [25][59][60][61][62][63][64][65][66][67][68][69].
Clinical trials exploring combinational immunotherapy in HNSCC are underway. The phase 3 randomized trial CheckMate 651 NCT02741570), which compared nivolumab and ipilimumab against EXTREME (platinum/5-fluorouracil/cetuximab) for R/M HNSCC, was unsuccessful in demonstrating OS improvement, although there was an association between elevated CPS and OS and durable response [70]. Other combination ICI therapy clinical trials have been largely unsuccessful (Table 1).
Table 1. Combination ICI Therapy Clinical Trials in HNSCC.
| Target | Combination | Phase | Trial | Intent | Outcome |
|---|---|---|---|---|---|
| PD-1, CTLA-4 | Nivolumab, Ipilimumab | 3 | NCT027441570 (CheckMate 651) [71] | Combination nivolumab + ipilimumab vs. EXTREME Regime (platinum/5-fluorouracil/cetuximab) for R/M HNSCC | Failed endpoint (OS). No difference between dual ICI blockade and EXTREME arm. Improvement in dual ICI arm if CPS > 20 (ns) |
| PD-L1, CTLA-4 | Durvalumab, Tremelimumab | 3 | NCT02551159 (KESTRAL) [72] | Combination durvalumab + tremelimumab vs. duravalumab monotherapy vs. SOC CT in R/M HNSCC | Results pending |
| PD-1, CTLA-4 | Nivolumab, Ipilimumab | 2 | NCT02823574 (CheckMate 714) [73] | Combination nivolumab + ipilimumab vs. nivolumab + ipilimumab placebo in R/M HNSCC | Failed ORR and OS endpoints. Subpopulation assessment ongoing. |
| PD-L1, CTLA-4 | Durvalumab, Tremelimumab | 3 | NCT02369874 (EAGLE) [74][75] | Combination durvalumab + tremelimumab vs. durvalumab monotherapy vs. SOC in R/M HNSCC | Failed to meet primary OS improvement endpoint |
Concurrent neoadjuvant and adjuvant delivery of ICIs has recently demonstrated benefits in surgically resectable advanced melanoma (Stage IIIB to IVC). In a recently completed Phase 2 randomized study (NCT03698019), neoadjuvant-adjuvant delivery of pembrolizumab was compared to an adjuvant alone in demonstrating an event-free survival of 72% in the neoadjuvant-adjuvant group compared to 49% in the adjuvant group after 2 years [76].
2.2. LAG-3
LAG-3 is expressed on activated human T-cells and natural killer cells and plays a similar role in T-cell regulation to CTLA-4 and PD-1 [77]. LAG-3 may represent an intrinsic resistance mechanism to PD-1 inhibitors due to its synergistic co-expression with PD-1 on exhausted T-cells [77]. To combat resistance, the FDA-approved drug opdualag® (combined LAG-3 and PD-1 inhibitor) became a first-line treatment for unresectable or metastatic melanoma in March 2022 [77]. Opdualag has shown success in clinical trials, more than doubling progression-free survival compared to melanoma patients treated with nivolumab alone [78].
2.3. Tim-3 and CD39
T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) is a co-inhibitory receptor expressed on IFN-γ-producing T-cells Tim-3. Studies by Liu et al. showed that Tim-3 is linked to immunosuppression in HNSCC and that targeting Tim-3 (with monoclonal antibodies) can enhance the anti-tumor immune response by reducing Tregs in HNSCC [79]. Similarly, the expression of the cell-surface ectonucleosidase CD39 in HNSCC positively correlates with tumor stage and predicts poor prognosis [80]. There are no approved inhibitors of Tim-3 or CD39, and opdualag has not yet been approved for HNSCC.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241411695
References
- Tam, S.; Yao, C.M.K.L.; Amit, M.; Gajera, M.; Luo, X.; Treistman, R.; Khanna, A.; Aashiq, M.; Nagarajan, P.; Bell, D.; et al. Association of Immunosuppression with Outcomes of Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 128–135.
- Szturz, P.; Vermorken, J.B. Treatment of Elderly Patients with Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2016, 6, 199.
- Pritchett, E.N.; Doyle, A.; Shaver, C.M.; Miller, B.; Abdelmalek, M.; Cusack, C.A.; Malat, G.E.; Chung, C.L. Nonmelanoma Skin Cancer in Nonwhite Organ Transplant Recipients. JAMA Dermatol. 2016, 152, 1348–1353.
- Gilbert, M.; Liang, E.; Li, P.; Salgia, R.; Abouljoud, M.; Siddiqui, F. Outcomes of Primary Mucosal Head and Neck Squamous Cell Carcinoma in Solid Organ Transplant Recipients. Cureus 2022, 14, e24305.
- Mowery, A.J.; Conlin, M.J.; Clayburgh, D.R. Elevated incidence of head and neck cancer in solid organ transplant recipients. Head Neck 2019, 41, 4009–4017.
- Mowery, A.; Conlin, M.; Clayburgh, D. Risk of Head and Neck Cancer in Patients with Prior Hematologic Malignant Tumors. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1121–1127.
- Wang, X.; Wang, H.; Zhang, T.; Cai, L.; Dai, E.; He, J. Diabetes and its Potential Impact on Head and Neck Oncogenesis. J. Cancer 2020, 11, 583–591.
- Zhong, W.; Mao, Y. Daily Insulin Dose and Cancer Risk among Patients with Type 1 Diabetes. JAMA Oncol. 2022, 8, 1356–1358.
- Batista, N.V.R.; Valdez, R.M.A.; Silva, E.; Melo, T.S.; Pereira, J.R.D.; Warnakulasuriya, S.; Santos-Silva, A.R.; Duarte, A.; Mariz, H.A.; Gueiros, L.A. Association between autoimmune rheumatic diseases and head and neck cancer: Systematic review and meta-analysis. J. Oral Pathol. Med. 2022, 52, 357–364.
- Li, C.M.; Chen, Z. Autoimmunity as an Etiological Factor of Cancer: The Transformative Potential of Chronic Type 2 Inflammation. Front. Cell Dev. Biol. 2021, 9, 664305.
- D’souza, G.; Carey, T.E.; William, W.N., Jr.; Nguyen, M.L.; Ko, E.C.; Riddell, J., IV; Pai, S.I.; Gupta, V.; Walline, H.M.; Lee, J.J.; et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2014, 65, 603–610.
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360.
- Dorsey, K.; Agulnik, M. Promising new molecular targeted therapies in head and neck cancer. Drugs 2013, 73, 315–325.
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108.
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008, 359, 1143–1154.
- Qiao, X.W.; Jiang, J.; Pang, X.; Huang, M.C.; Tang, Y.J.; Liang, X.H.; Tang, Y.L. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721.
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22.
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72.
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma–An update. CA Cancer J. Clin. 2015, 65, 401–421.
- Mehra, R.; Cohen, R.B.; Burtness, B.A. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin. Adv. Hematol. Oncol. 2008, 6, 742–750.
- Sundvall, M.; Karrila, A.; Nordberg, J.; Grénman, R.; Elenius, K. EGFR targeting drugs in the treatment of head and neck squamous cell carcinoma. Expert Opin. Emerg. Drugs 2010, 15, 185–201.
- Sacco, A.G.; Worden, F.P. Molecularly targeted therapy for the treatment of head and neck cancer: A review of the ErbB family inhibitors. Onco Targets Ther. 2016, 9, 1927–1943.
- Addeo, R.; Ghiani, M.; Merlino, F.; Ricciardiello, F.; Caraglia, M. CheckMate 141 trial: All that glitters is not gold. Expert Opin. Biol. Ther. 2019, 19, 169–171.
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867.
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965.
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167.
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928.
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355.
- Zandberg, D.P.; Algazi, A.P.; Jimeno, A.; Good, J.S.; Fayette, J.; Bouganim, N.; Ready, N.E.; Clement, P.M.; Even, C.; Jang, R.W.; et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur. J. Cancer 2019, 107, 142–152.
- Jamieson, L.; Forster, M.D.; Zaki, K.; Mithra, S.; Alli, H.; O’Connor, A.; Patel, A.; Wong, I.C.K.; Chambers, P. Immunotherapy and associated immune-related adverse events at a large UK centre: A mixed methods study. BMC Cancer 2020, 20, 743.
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728.
- Kessler, R.; Pandruvada, S. Immune-related adverse events following checkpoint inhibitor treatment in head and neck cancers: A comprehensive review. Oral Oncol. Rep. 2023, 6, 100036.
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 blockade in cancer immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674.
- Park, R.; Lopes, L.; Saeed, A. Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: Systematic review and meta-analysis. Clin. Transl. Oncol. 2021, 23, 100–109.
- Griewing, L.M.; Schweizer, C.; Schubert, P.; Rutzner, S.; Eckstein, M.; Frey, B.; Haderlein, M.; Weissmann, T.; Semrau, S.; Gostian, A.-O. Questionnaire-based detection of immune-related adverse events in cancer patients treated with PD-1/PD-L1 immune checkpoint inhibitors. BMC Cancer 2021, 21, 314.
- Schweizer, C.; Schubert, P.; Rutzner, S.; Eckstein, M.; Haderlein, M.; Lettmaier, S.; Semrau, S.; Gostian, A.-O.; Frey, B.; Gaipl, U.S. Prospective evaluation of the prognostic value of immune-related adverse events in patients with non-melanoma solid tumour treated with PD-1/PD-L1 inhibitors alone and in combination with radiotherapy. Eur. J. Cancer 2020, 140, 55–62.
- Matsuki, T.; Okamoto, I.; Fushimi, C.; Takahashi, H.; Okada, T.; Kondo, T.; Sato, H.; Ito, T.; Tokashiki, K.; Tsukahara, K. Real-world, long-term outcomes of nivolumab therapy for recurrent or metastatic squamous cell carcinoma of the head and neck and impact of the magnitude of best overall response: A retrospective multicenter study of 88 patients. Cancers 2020, 12, 3427.
- Les, I.; Martínez, M.; Pérez-Francisco, I.; Cabero, M.; Teijeira, L.; Arrazubi, V.; Torrego, N.; Campillo-Calatayud, A.; Elejalde, I.; Kochan, G.; et al. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers 2023, 15, 1629.
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Masteller, E.L.; Chuang, E.; Mullen, A.C.; Reiner, S.L.; Thompson, C.B. Structural analysis of CTLA-4 function in vivo. J. Immunol. 2000, 164, 5319–5327.
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106.
- Qiao, Y.; Liu, C.; Zhang, X.; Zhou, Q.; Li, Y.; Xu, Y.; Gao, Z.; Xu, Y.; Kong, L.; Yang, A.; et al. PD-L2 based immune signature confers poor prognosis in HNSCC. Oncoimmunology 2021, 10, 1947569.
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499.
- Hino, R.; Kabashima, K.; Kato, Y.; Yagi, H.; Nakamura, M.; Honjo, T.; Okazaki, T.; Tokura, Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010, 116, 1757–1766.
- Rasmussen, M.; Durhuus, J.A.; Nilbert, M.; Andersen, O.; Therkildsen, C. Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 329.
- De Sousa Linhares, A.; Leitner, J.; Grabmeier-Pfistershammer, K.; Steinberger, P. Not All Immune Checkpoints Are Created Equal. Front. Immunol. 2018, 9, 1909.
- Haibe, Y.; El Husseini, Z.; El Sayed, R.; Shamseddine, A. Resisting Resistance to Immune Checkpoint Therapy: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 6176.
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693.
- García-Aranda, M.; Redondo, M. Targeting Protein Kinases to Enhance the Response to anti-PD-1/PD-L1 Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2296.
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089.
- Yan, M.; Zheng, M.; Niu, R.; Yang, X.; Tian, S.; Fan, L.; Li, Y.; Zhang, S. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front. Cell Dev. Biol. 2022, 10, 938289.
- Xiao, M.; He, J.; Yin, L.; Chen, X.; Zu, X.; Shen, Y. Tumor-Associated Macrophages: Critical Players in Drug Resistance of Breast Cancer. Front. Immunol. 2021, 12, 799428.
- Ma, T.; Renz, B.W.; Ilmer, M.; Koch, D.; Yang, Y.; Werner, J.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Solid Tumors. Cells 2022, 11, 310.
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 2854.
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15.
- Zhong, W.; Wang, Q.; Shen, X.; Du, J. The emerging role of neutrophil extracellular traps in cancer: From lab to ward. Front. Oncol. 2023, 13, 1163802.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723.
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159.
- Noji, R.; Tohyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Michi, Y.; Tasaki, A.; Ohno, K.; Ariizumi, Y.; et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers 2022, 14, 3476.
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940.
- Jing, F.; Wang, J.; Zhou, L.; Ning, Y.; Xu, S.; Zhu, Y. Bioinformatics analysis of the role of CXC ligands in the microenvironment of head and neck tumor. Aging 2021, 13, 17789–17817.
- de Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148.
- Xu, Q.; Wang, C.; Yuan, X.; Feng, Z.; Han, Z. Prognostic Value of Tumor-Infiltrating Lymphocytes for Patients with Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2017, 10, 10–16.
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452.
- Kao, H.F.; Liao, B.C.; Huang, Y.L.; Huang, H.C.; Chen, C.N.; Chen, T.C.; Hong, Y.J.; Chan, C.Y.; Chia, J.S.; Hong, R.L. Afatinib and Pembrolizumab for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (ALPHA Study): A Phase II Study with Biomarker Analysis. Clin. Cancer Res. 2022, 28, 1560–1571.
- Mascarella, M.A.; Mannard, E.; Silva, S.D.; Zeitouni, A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck 2018, 40, 1091–1100.
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468.
- Chen, Y.; Li, Z.Y.; Zhou, G.Q.; Sun, Y. An Immune-Related Gene Prognostic Index for Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2021, 27, 330–341.
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021, 184, 596–614.e14.
- Argiris, A.; Harrington, K.; Tahara, M.; Ferris, R.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Nin, R.M.; Saba, N. LBA36 Nivolumab (N)+ ipilimumab (I) vs EXTREME as first-line (1L) treatment (tx) for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): Final results of CheckMate 651. Ann. Oncol. 2021, 32, S1310–S1311.
- Argiris, A.; Gillison, M.; Ferris, R.; Harrington, K.; Sanchez, T.; Baudelet, C.; Geese, W.; Shaw, J.; Haddad, R. A randomized, open-label, phase 3 study of nivolumab in combination with ipilimumab vs extreme regimen (cetuximab + cisplatin/carboplatin + fluorouracil) as first-line therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck-CheckMate 651. Ann. Oncol. 2016, 27, vi350.
- Seiwert, T.Y.; Weiss, J.; Baxi, S.S.; Ahn, M.-J.; Fayette, J.; Gillison, M.L.; Machiels, J.-P.H.; Takahashi, S.; Melillo, G.; Franks, A. A phase 3, randomized, open-label study of first-line durvalumab (MEDI4736) ± tremelimumab versus standard of care (SoC; EXTREME regimen) in recurrent/metastatic (R/M) SCCHN: KESTREL. J. Clin. Oncol. 2016, 34, TPS6101.
- Harrington, K.J.; Ferris, R.L.; Gillison, M.; Tahara, M.; Argiris, A.; Fayette, J.; Schenker, M.; Bratland, Å.; Walker, J.W.T.; Grell, P.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab vs Nivolumab Alone for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: The Phase 2 CheckMate 714 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 779–789.
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950.
- Licitra, L.F.; Haddad, R.I.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.-E.; Clement, P.M.; Mesia, R.; Kutukova, S.I.; Zholudeva, L. EAGLE: A phase 3, randomized, open-label study of durvalumab (D) with or without tremelimumab (T) in patients (pts) with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37, 6012.
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823.
- Chocarro, L.; Bocanegra, A.; Blanco, E.; Fernández-Rubio, L.; Arasanz, H.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro-Hermida, S.; Vera, R.; et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells 2022, 11, 2351.
- Albrecht, L.J.; Livingstone, E.; Zimmer, L.; Schadendorf, D. The Latest Option: Nivolumab and Relatlimab in Advanced Melanoma. Curr. Oncol. Rep. 2023, 25, 647–657.
- Liu, J.F.; Wu, L.; Yang, L.L.; Deng, W.W.; Mao, L.; Wu, H.; Zhang, W.F.; Sun, Z.J. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 2018, 37, 44.
- Mandapathil, M.; Boduc, M.; Roessler, M.; Güldner, C.; Walliczek-Dworschak, U.; Mandic, R. Ectonucleotidase CD39 expression in regional metastases in head and neck cancer. Acta Oto-Laryngol. 2018, 138, 428–432.
This entry is offline, you can click here to edit this entry!
