The application of metallic nanoparticles as a novel therapeutic tool has significant potential to facilitate the treatment and diagnosis of mitochondria-based disorders. Subcellular mitochondria have been trialed to cure pathologies that depend on their dysfunction. Nanoparticles made from metals and their oxides (including gold, iron, silver, platinum, zinc oxide, and titanium dioxide) have unique modi operandi that can competently rectify mitochondrial disorders.
- metallic nanoparticles
- mitochondrial dysfunction
- antioxidants
- reactive oxygen species
- calcium homeostasis
- biocompatibility
1. Introduction
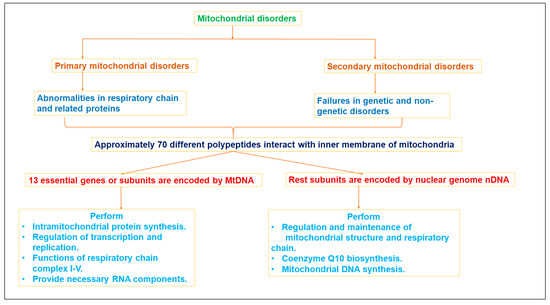
2. Mitochondrial Dysfunction and Diseases
|
Disease |
Mitochondrial Abnormalities |
Management |
Ref. |
|---|---|---|---|
|
Cardiovascular diseases |
Impaired mitochondrial electron transport chain due to elevated LDL, greater ROS production disturbed cardiac tension, altered cytosolic Ca2+ flux, ischemia-reperfusion injury, and other diseases (such as diabetes mellitus) |
Control fatty acids and cholesterol level. Regulate enzymes i.e., mitochondrial creatin phosphate, creatin kinase, and ATP synthase. Modulate Ca2+ concentration in myocardium. |
[8] |
|
Diabetes |
MtDNA mutation Delayed Electron transport chain Increased beta oxidation and lipid accumulation Higher ROS overproduction Disturbed insulin signal pathway Increased intracellular glucose content, and constrained metabolism of glucose |
Prevention of ROS and lipid peroxidation. Reverse MtDNA change. Enhance glucose metabolism by inhibiting acetyl-CoA in mitochondria. |
[9] |
|
Kidney |
Mitochondrial DNA mutation (complex I–V) Hypomagnesemia Hypokalemia Hypoparathyroidasim Uremic toxins Kidney diseases |
Manage Erythropoetin signaling for normal mitochondrial biogenesis and metabolism. Hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors, Nrf2-activating triterpenoid, sodium–glucose transporter 2 (SGLT2) inhibitors, and control of carnitine level |
[10] |
|
Alzheimer’s disease |
Aggregated Aβ peptide bond with a component that controls mitochondrial permeability ‘cyclophilin D’ Resultant reduction in membrane potential due to opening of pores. Free energy and ROS generated by beta amyloid peptide. Leads to MtDNA mutation and neuronal toxicity. |
Inhibition ofAβ peptide clusters binding with cyclophilin D. Restoration of MtDNA and enzyme replacement Inhibit cytochrome c and caspase activity Decrease mitochondrial fission |
[11] |
|
Cancer |
Enhanced mitochondrial complex I activity. Mutations in oncogenes Expression of oncoproteins (IDH 1 and 2, SDH and FH) Apoptosis induced factors released. Defective oxidative phosphorylation Hypoxic milieu proliferate condition |
Isocitrate dehydrogenase (IDH) 1 and 2 inhibitors Oxidative phosphorylation suppression Antioxidants Mitochondrial complex I and V inhibitors Lactate dehydrogenase (LDH) inhibitors |
[12] |
3. Therapeutic Approaches
|
Therapeutics |
Approach |
Model |
Outcome |
Ref. |
|---|---|---|---|---|
|
Upsurging ATP levels |
||||
|
Inosine and Febuxostat |
To increase ATP level and hypoxanthine in peripheral blood |
Patients with homoplasmic and heteroplasmic mutations |
After oral administration, brain natriuretic peptide (specific marker for heart failure) was reduced up to 31%. Moreover, 3.1-fold insulinogenic index was improved, suggesting a promising action of the given treatment. |
[13] |
|
Stimulating mitochondrial biogenesis |
||||
|
Bezafibrate and AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide |
To initiate biogenesis and activate AMP protein kinase/PGC-1α-dependent pathway |
Double recombinant mice overexpressing PGC-1α in skeletal muscles |
Stimulation of PPAR/AMPK/PGC-1 alpha increased mitochondrial biogenesis, which monitors the homeostatic pathway and motor improvement in Sco2(KO/KI) animal model |
[14] |
|
5-Aminoimidazole-4-carboxamide ribotide (AICAR) |
ATP content and mitochondrial growth were aimed without disturbing membrane potential |
CI deficient fibroblasts, such as NDUFS2 and C20ORF7 |
Fluorescence microscopy detailed the activation of AMP protein kinase with AICAR |
[15] |
|
Nicotinamide riboside (NAD+ precursor) |
Effect of nicotinamide in pharmacokinetic parameters and NAD+ level in blood for the treatment of genetic or acquired mitochondrial abnormalities |
Impaired mitochondrial murine model |
Orally administered nicotinamide riboside was well tolerated, and an increased mean steady state concentration (Css, p = 0.03) two times greater than the baseline NAD+ concentration in blood was observed, whereas average circulating level of NAD+ at day 1 was 27 ± 6 µM. |
[16] |
|
Modulation of the Nitric acid or cGMP/PKG pathway |
||||
|
L-arginine (Nitric acid precursor) |
To reduce capacity for nitric acid-based vasodilation |
MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) patients |
Obtained data suggested that prepared L-Arg infusion was most effective when administered within 4 hrs of the onset of brain disorder symptoms in the acute phase of MELAS sufferers |
[17] |
|
Neural progenitor cells (NPC) |
To preserve parental mtDNA and show metabolic shift toward oxidative phosphorylation |
Homoplasmic mutation in the mitochondrial gene (MT-ATP6) |
Human induced pluripotent stem cells originated NPC, providing a potential tool for mtDNA targeted drug screening to avoid nervous system disorder. |
[18] |
|
Antioxidant therapy |
||||
|
N-acetyl cysteine |
Supplementation with N-acetyl cysteine to modify the mitochondrial respiratory chain function |
MELAS patients containing common mutation, i.e., m.3243A>G, m.8344A>G |
Supplementation with N-acetyl cysteine improved 2-thiomodificationof tRNA, thus regulating protein synthesis. |
[19] |
|
Cysteamine bitartrate |
Enhancement of glutathione biosynthesis for the reduction of oxidative stress associated with mitochondrial diseases |
Zebrafish model and Caenorhabditis elegans model carrying Complex I defect |
Cysteamine bitartrate improved mitochondrial membrane potential in nephropathic cystinosis and cured multiple RC complex diseases in FBXL4 human at 10 to 100 μm concentrations. |
[20] |
|
Inclusion of Redox-Active Molecules |
||||
|
Tc99m-HMPAO |
To explore Tc99m-HMPAO for determination of glutathione/protein thiol levels in cerebral blood flow |
Pediatric mitochondrial diseased patients |
The patients showed improvement in Newcastle score (n = 5, p = 0.028), confirming the potential of Tc99m-HMPAO as a bioimaging marker for the oxidative state of brain. |
[21] |
|
EPI-743 |
Management of cellular oxidative stress in mitochondrial respiratory chain disease |
5-month-old girl suffering from Leigh syndrome |
EPI-743 (a potent stress protectant) exhibited improvement in mitochondrial associated issues, i.e., improvement in eye, motor, and bowel movements |
[22] |
|
Monitoring of mitochondrial dynamics |
||||
|
Cytotoxic Necrotizing Factor-1 |
To control mitochondrial impairment and cell damage |
Patient sufferer from m.8344A>G gene |
CNF-1 triggered energetic content of mitochondria via activation of actin cycloskeleton in Myoclinic epilepsy with ragged red fibers (MERRF) and increased mitochondrial marker tomo20 |
[23] |
|
Modulating mitochondrial autophagy |
||||
|
Rapamycin |
Inhibition of mTOR in mitochondrial defect in Leigh syndrome |
Ndufs4 wild type mouse |
Rapamycin slowed down neurological symptoms and minimized neuroinflammation in brain lesions. It also slowed the formation of glycolytic intermediates. |
[24] |
|
NADH dehydrogenase Ndi1 |
To replace mitochondrial complex 1 for reoxidation of NADH intramitochondrially. |
Transgenic strains of Drosophila |
Overexpression of NDI1 relieves aging and manages production of ROS. Deposition of these oxidative damaged markers is declined in aged flies. |
[25] |
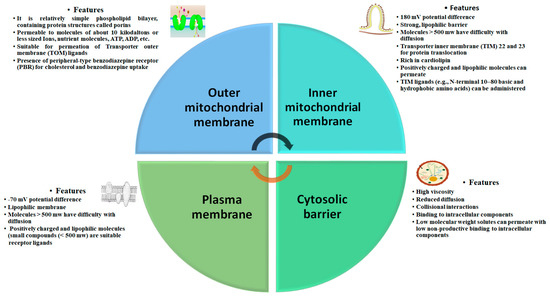
4. Biological Barrier and Toxicity
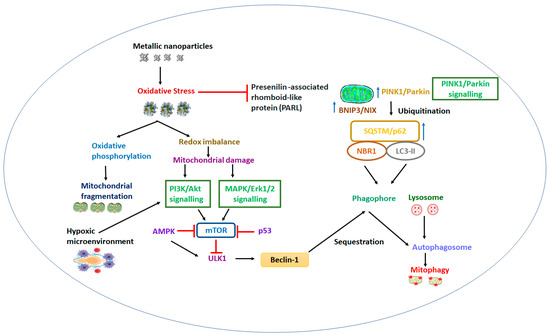
5. Nanoengineered Mitochondria Targeted Approaches
|
Nanodrug Delivery System |
Purpose |
Model |
Outcomes |
Relevance |
Ref. |
|---|---|---|---|---|---|
|
Topotecan loaded liposome |
Mitochondrial targeted system to overcome resistant related metastases |
Multi drug Resistant MCF-7/ADR cell xenografts |
Mitochondrial targeted liposomes were 64.84 nm with −0.52 ± 0.08 mV membrane potential. Encapsulation efficiency was ≥95%. They were stable in physiological blood system and exhibited minimal leakage. The system led to release cytochrome C, stimulated caspase 9 and 3, and exhibited superior inhibitory action on the resistant B16 melanoma metastatic mice. |
Topotecan localized in mitochondria that exhibited potent inhibitory action on the on the resistant B16 metastatic melanoma. |
[44] |
|
Paclitaxel loaded triphenylphosphine nanomicelles |
Inhibition of antiapoptotic Bcl-2 |
Drug-resistant breast cancer-bearing mouse model with lung metastasis (A549/ADRcells) |
The nanomicelles were small (142 ± 8.35 nm) with PDI 0.235 and negative zeta potential (−24.65 ). This system significantly hampered A549/ADR cells and deposited over mitochondria surface. Inhibition of Bcl-2 led to release cytochrome C and triggered caspase 3 and 9, mediating mitochondrial outer membrane permeabilization. |
Positively charged nanomicelles adhered inside mitochondria and exhibited apparent multidrug resistant tumor targeting efficacy |
[45] |
|
Curcumin loaded polymeric and lipid nanosuspensions |
To neutralize generation of reactive oxygen species due to disturbance of signal proteins in mitochondria. |
Olfactory ensheathing cells |
Uniform and spherical polymeric and lipid nanosuspensions were of mean sizes 338 nm and 127 nm, respectively, with negatively charged zeta potential. The nanosuspensions were quite stable for more than 135 days. Improved cell viability suggested potential incorporation of curcumin in nanosuspension against hypoxic cells of Olfactory ensheathing cells at the concentration of 5 µM. |
Potential intranasal polymeric and lipid nanosuspention was advised for neuroprotective action due to antioxidant property of curcumin. |
[46] |
|
Hydroxyl-terminated polyamidoamine (PAMAM)-N-acetyl cysteine dendrimers |
Mitochondrial targeted delivery in oxidative stress-induced glial cell |
Rabbit traumatic brain injury (TBI) model |
Significantly high localization of drug in mitochondria than nonmodified dendrimer due to potential for attenuation of oxidative stress. Systemic administration in TBI model of rabbit exhibited capability to penetrate BBB and target glial cells due to localization in the white matter of the injured hemisphere. |
Colocalization of dendrimer in mitochondria of glial cells in traumatic brain injury |
[47] |
|
Osthole nanoemulsion (OST-NE) |
Regulation of apoptosis pathway and mitochondrial oxidative stress. |
Alzheimer’s disease model mice |
Intranasal delivery of osthole nanoemulsion (mean particle size 2.33 nm) enhanced bioavailability, regulated cholinergic system, maintained mitochondrial potential, and inhibited apoptosis and oxidative stress. |
OST-NE lessened upstream modulator Bax, that depolarized mitochondrial membrane potentail and exhibited antiapoptotic effect or neuroprotective action in Alzheimer disease |
[48] |
|
Momordica charantia silver nanoparticles |
Uphold mitochondria biogenesis and enhanced the expression of PPARϒ, an energy metabolism coordinator |
Pancrease of diabetic rats |
Silver nanoparticles possessed irregular/uneven surface and diameter. Contained Momordica charantia enhanced glucose sensitivity in the dibetic rat model at a lower dose of 50 mg/kg by slowing down JAK/STAT and AKT/PI3K pathways in mitochondria. |
Developed nanoparticles promoted glucose uptake and insulin secretion via improving mitochondrial biogenesis in pancreas of diabetic rats. |
[49] |
|
Dequalinium embedded DQAsomes |
Dicationic amphiphilic was investigated for affinity towards binding with mitochondria DNA. |
Plasmid DNA firefly luciferase |
Developed DQAsomes created a liposome-like aggregate system in aqueous media that were able to bind with DNA and had efficiency to transfect cells compared to Lipofectin™ reagent. |
DQAsomes selectively accumulated in mitochondria of cancerous cells, and suggeted potential use as a nonviral transfection vector in gene delivery system. |
[50] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules28124701
