Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chronic kidney disease (CKD) is one of the most common complications of diabetes mellitus and an independent risk factor for cardiovascular disease. Despite guideline-directed therapy of CKD in patients with type 2 diabetes, the risk of renal failure and cardiovascular events still remains high, and diabetes remains the leading cause of end-stage kidney disease in affected patients. To date, current medications for CKD and type 2 diabetes mellitus have not reset residual risk in patients due to a high grade of inflammation and fibrosis contributing to kidney and heart disease.
- type 2 diabetes mellitus
- mineralocorticoid receptor antagonists
- inflammation
1. Introduction
Despite current therapeutic options, patients with diabetic renal disease still have a residual risk of progression to end-stage renal disease with a related increased incidence of cardiovascular events. Beyond the metabolic and hemodynamic aspects, which are well controlled by currently available molecules (from inhibitors of the renin–angiotensin–aldosterone system, RAASi, to the co-receptor sodium/glucose transporter type 2 inhibitors, SGLT2i), are chronic inflammatory patterns. These are little explored but extremely important in determining renal and cardiovascular outcomes. Chronic inflammatory patterns present in patients can evolve into fibrosis, both renal and cardiac.
As of 2019, the American Diabetes Association (ADA) has recommended the use of SGLT2i in combination with angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs) with the purpose of slowing the progression of renal disease and reducing the impact of cardiovascular events in patients with diabetic nephropathy [1].
Since there is evidence of mineralocorticoid receptor (MR) hyperactivation in patients with chronic kidney disease and diabetes, which could lead to an increased rate of inflammation first and fibrosis later, the blockade of receptor activity could contribute to the lowering of residual cardiorenal risk in such a patient population [2].
The anti-proteinuric and anti-fibrotic effect of spironolactone, a steroid-type mineralocorticoid receptor antagonist (MRA), has long been known; however, such effectiveness has been burdened by a high incidence of side effects (gynecomastia and hyperkalemia above all) [2].
Finerenone, in contrast to spironolactone and eplerenone, is a non-steroidal type MRA (hence the absence of gynecomastia as a side effect) with a high affinity (significantly higher than both spironolactone and eplerenone) for the mineralocorticoid receptor. It exhibits a balanced tissue distribution between the heart and kidney and, most importantly, is associated with a lower risk of hyperkalemia, as evidenced by the results of phase I and phase II clinical trial programs (Figure 1) [3].
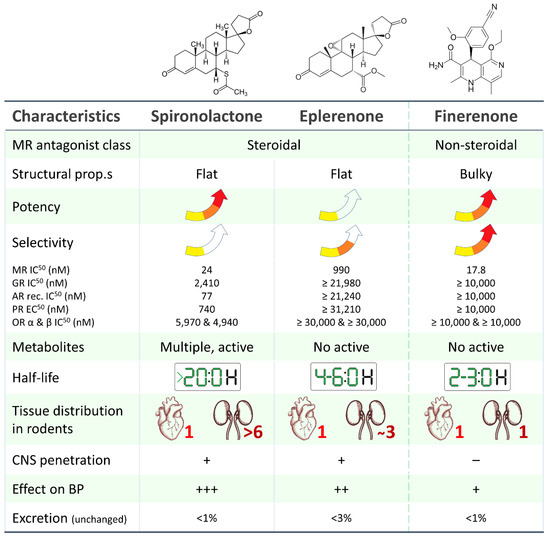
Figure 1. Main pharmacological and pharmacokinetic characteristics of steroidal (spironolactone, eplerenone) and non-steroidal (finerenone) mineralocorticoid receptor antagonists [4][5][6][7][8][9]. MR: mineralocorticoid receptor, AR: androgen receptor, GR: glucocorticoid receptor, PR: progesterone receptor, OR: oestrogen receptor.
2. Finerenone: Why Another MRA?
The mineralocorticoid receptor (MR) is a ligand-induced transcription factor expressed in many tissues, including the kidney, heart, and blood vessels. When aldosterone binds MR, it changes its conformation and translocates from the cytoplasm into the nucleus, binding specific hormone-response elements and recruiting transcriptional cofactors which act in the transcription or repression of target genes [10]. Nevertheless, MR is also responsible for a “non-genomic” activity modulating target molecules and pathways [11].
The role of MR is well characterized in the distal nephron on aldosterone-sensitive kidney epithelial cells that express the 11b-hydroxysteroid dehydrogenase type 2 enzyme, which confers higher specificity of the MR for aldosterone versus cortisol. In these cells, MR activation is primarily involved in the regulation of blood pressure and sodium retention [2]. MR receptor is also expressed in kidney non-epithelial cells and in several other tissues including adipocytes, vascular smooth muscle cells (VSMCs), immune cells, and heart cells, in which it may be involved in regulation of cardiomyocyte growth and cardiac electrophysiology. In non-epithelial cells, MR is widely activated also by cortisol and its upregulation could stimulate fibrosis and inflammation, resulting in progression of renal and cardiovascular disease (Figure 2) [12].
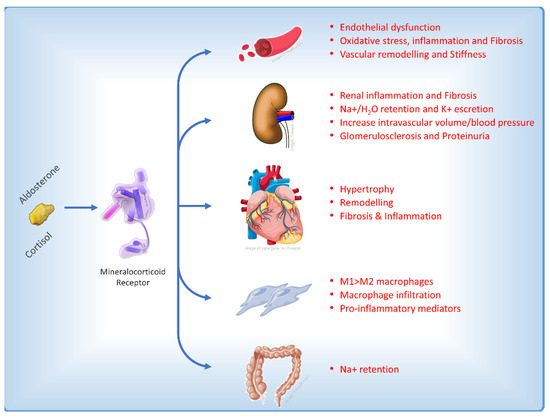
Figure 2. Main effects on different cellular types and pathophysiological pathways affecting the heart induced by the activation of the mineralocorticoid receptor [13].
In CKD experimental models, it was observed that aldosterone increased reactive oxygen species [14] and promoted mesangial apoptosis [15] and collagen synthesis [16]. This resulted in inflammation and cortical and medullary fibrosis. Injury of podocytes, a critical event in diabetic nephropathy, is prevented in mice by MR antagonism with eplerenone [17]. Autophagy, another essential process for podocyte maintenance and homeostasis, is also disrupted in CKD associated with type 2 diabetes (T2D) and spironolactone treatment increased podocytes autophagy in diabetic rats and restored podocytes autophagy under mechanical stress [18]. In endothelial cells, aldosterone promotes oxidative injury and endothelial disfunction [19][20] as MR activation is involved in vascular calcification and fibrosis, stiffness, and inflammation [21]. Furthermore, the activation of mineralocorticoid receptors (MR) has been found to have additional pathological effects on the immune system and pro-inflammatory cells. This includes the stimulation of the infiltration of pro-inflammatory M1 macrophages as opposed to M2 macrophages, increased proliferation of fibroblasts, the production of molecules that promote fibrosis [22], and a reduction in nitric oxide levels due to the influence of MR on the expression of the epithelial sodium channel in the endothelium [23].
In streptozotocin-induced diabetic rats and db/db mice, MR blockade with spironolactone, eplerenone, and finerenone reduces collagen deposition in glomerular, tubulointerstitial, and perivascular areas as well as decreased albuminuria [24][25][26].
In order to prevent severe comorbidities associated with T2D, such as nephropathy and end-stage renal disease (ESRD) and cardiovascular morbidity and mortality, preserving organ integrity and function is a challenge in patients with T2D [2].
The first steroidal MR antagonist (MRA), spironolactone, was developed as an antihypertensive drug with the intention to prevent sodium retention and decrease blood volume. However, because of its activities on progesterone and other nuclear receptors, it was observed that spironolactone causes relevant side effects, such as gynecomastia, impotence, and menstrual abnormalities. These side effects were at least partially ameliorated by the second-generation of steroidal MRA eplerenone [27]. Indeed, although it was observed in randomized control trials that steroidal MRAs (spironolactone and eplerenone) reduce the risk of hospitalization and cardiovascular death in patients with HF and reduced ejection fraction (HFrEF) [28][29], and received class IA recommendation by international clinical guidelines for the treatment of HFrEF [30], they are still underused, especially in patients with impaired kidney function [31], because of the risk of both acute kidney injury (AKI) and hyperkalemia. Furthermore, spironolactone is recommended by guidelines as an optimal fourth-line therapy in patients with resistant hypertension and estimated glomerular filtration rate (eGFR) >45 mL/min/1.73 m2 and serum K ≤ 4.5 mEq/L [32][33], but the risk of hyperkalemia represents the limit of use of MRAs also in this context [34][35].
As previously mentioned, MR activation concurs in the pathophysiology of CKD [36], and aldosterone is supposed to be one among main actors. Aldosterone levels were shown to start to increase when the glomerular filtration rate (GFR) is reduced by 50% [37] and another study showed that aldosterone levels are elevated up to fourfold in patients with a mean GFR of 27 mL/min per 1.73 m2 [38]. Additionally, during long-term blockade of the renin-angiotensin system (RAS), aldosterone breakthrough is seen [39]. The correlation between aldosterone levels, eGRF decline, and the aldosterone breakthrough phenomenon therefore supports aldosterone as a primary target for intervention in patients with CKD and T2D who are receiving a maximum-tolerated dose of ACEis or ARBs, shifting from RAS blockade to a renin-angiotensin-aldosteron system (RAAS) blockage. Several meta-analyses on steroidal MRAs in diabetic CKD reported an improvement in urinary albumin–creatinine ratio (UACR) but with a dramatic increased incidence of hyperkalemia [40][41]. Hou analyzed 16 studies of spironolactone added to routine antidiabetic, renoprotective, and antihypertensive treatment lasting 2–18 months and demonstrated a significant reduction of more than 60% in end-of-treatment 24-h urinary albumin/protein excretion, with the major concern of a more than a fivefold increased risk for hyperkalemia, particularly in those with impaired renal function [40]. Additionally, a 72-week intervention study with 4 arms combining irbesartan 150 or 300 mg daily with spironolactone 20 mg or placebo confirmed a long-term antiproteinuric effect (up to 30%) when combination was used [42]. More recently, prevention of CKD with spironolactone versus placebo was tested in the 3-year randomized PRIORITY study (Proteomic Prediction and Renin–Angiotensin–Aldosterone System Inhibition Prevention of Early Diabetic Nephropathy in Type 2 Diabetic Participants with normoalbuminuria). The study included 1777 patients with T2D and increased normal-to-mild albuminuria, of whom 218 had a high risk of CKD, as determined from a urinary proteomics-based risk pattern for CKD. To reduce the risk of hyperkalemia, only patients with a GFR > 45 mL/min/1.73 m2 were included. The urinary proteomic pattern predicted progression of both albuminuria and development of CKD stage 3b, and spironolactone was not able to prevent progression. Possible reasons for this result are the lack of statistical power, the short duration of the trial, or that the enrolled population was in too early a stage of disease for this mode of action to be effective [43].
Eplerenone, less potent but more selective than spironolactone, has documented benefits in HFrEF [29] and was considered a promising molecule for treating CKD in T2D patients because of the lack of hormonal side effects and antiproteinuric effects similar to those seen with spironolactone [44]. However, the increase in potassium levels resulted in a recommendation against eplerenone in T2D and CKD (US and UK labels).
Long-term studies with steroidal MRAs that aimed to prevent progression of established CKD have not been carried out, and therefore, whether the beneficial effect on albuminuria translates into prevention of ESKD is not known.
Given the beneficial effect on proteinuria but considering hyperkalemia and the sexual side effects of steroidal MRAs, the efforts go towards the discovery and development of new non-steroidal MRAs (NS-MRAs) that are highly selective for MR, and at least equally effective than spironolactone and with a better safety profile.
Finerenone is the first developed novel NS-MRA characterized by a unique binding mode (bulky) that determines high potency and selectivity for the MR, inhibits binding of both aldosterone and cortisol, and reduces recruitment of transcriptional cofactors in both the bound and not-bound conformational state of MR [2]. Indeed, the main difference among finerenone and spironolactone and eplerenone is the modulation of different transcriptional cofactors and expression/inhibition of a different genes profile.
Differently from steroidal MRAs, finerenone has also tissue distribution (equal distribution between the heart and kidney), pharmacokinetic properties (a short half-life and no active metabolites), a greater MR selectivity than spironolactone, and a higher receptor binding affinity than eplerenone [6][45][46].
Preclinical studies provided preliminary evidence that finerenone modifies tissue remodeling by exerting anti-inflammatory, antifibrotic and antiproliferative actions on both the heart and the kidney [46][47].
The phase II clinical trials (ARTS, ARTS-DN, ARTS-HF) have shown a dose-dependent reduction in albuminuria with finerenone and a side-effects profile at least similar with the placebo or comparator, as well as smaller treatment-induced increases in serum potassium levels with finerenone compared with spironolactone [48][49][50]. ARTS (Miner Alocorticoid Receptor antagonist Tolerability Study) evaluated various doses of finerenone for 28 days in 393 patients with T2D, CKD, and chronic HF with a reduced ejection fraction. Compared with spironolactone, a comparable reduction occurred in N-terminal pro-brain natriuretic peptide and albuminuria, with a smaller increase in serum potassium, and a significantly lower rate of hyperkalemia (5.3% vs. 12.7%) and renal impairment (3.8% vs. 28.6%) was observed [48]. The ARTS-DN (ARTS in Diabetic Nephropathy) study evaluated the effect on albuminuria of different doses of finerenone and a placebo for 90 days in 823 patients with T2D and CKD on top of RAS inhibitor therapy. Treatment with finerenone led to a dose-dependent reduction in albuminuria (21–38%). Adverse events were comparable to those observed with the placebo. Hyperkalemia and subsequent discontinuation of the study drug occurred in 1.8% of patients receiving finerenone, compared with no patients on placebo. In addition, no differences in the incidence of an eGFR decrease of 30% were seen between the groups [49]. These data support the assumption that the antiproteinuric effect can be maintained with a limited effect on serum potassium. Finally, similar results were achieved in the ARTS-HF (ARTS in Heart Failure) study which evaluated finerenone versus eplerenone in subjects with T2DM, a worsening of chronic heart failure, and with or without CKD [50].
Furthermore, in large phase III trials involving patients with CKD and T2DM (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIGARO-DKD)), finerenone significantly improved long-term kidney and cardiovascular outcomes and albuminuria as compared with the placebo, on top of RAS inhibition [51][52]. Although hyperkalemia was increased, values that required permanent discontinuation of finerenone were very low (2.3% for FIDELIO-DKD and 1.2% for FIGARO-DKD) These companion studies have been the first to test if the antiproteinuric effect of aldosterone blockade translates safely into prevention of progression of kidney disease and prevention of CV events.
CKD and diabetes independently increase the risk of atrial fibrillation (AF), as well as the risk of adverse CV outcomes in patients with AF; therefore, it is intriguing that in the FIDELIO-DKD study, new-onset AF occurred in 82 patients (3.2%) treated with finerenone versus 117 patients (4.5%) receiving the placebo (HR = 0.71; 95% CI = 0.53–0.94; P = ¼ 0.016) [53].
Finally, FIDELITY (finerenone in Chronic Kidney Disease and Type 2 Diabetes and combined FIDELIO-DKD and FIGARO-DKD trial program analysis) is the prespecified pooled-analysis of both phase III trials that included 13,026 patients with a mean follow-up of 3 years. Both composite cardiovascular (CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure) and kidney (kidney failure, sustained > or =57% decrease in eGFR from baseline over > or =4 weeks, or renal death) outcomes were significantly reduced by finerenone (14% and 23%, respectively) with a really low incidence of permanent drug discontinuation due to hyperkalemia events (1.7%) [2]. In summary, all reported evidence underlines both the clinical need of MR inhibition in the treatment of CKD in T2DM and the possibility that new NS-MRAs represent an effective tool with a better handling and risk/benefit ratio.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12123992
References
- American Diabetes Association. 11. Micorvascular complications and foot care standards of medical care in diabetes-2020. Diabetes Care 2020, 43, S135–S151.
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161.
- Agarwal, R.; Anker, S.D.; Bakris, G.; Filippatos, G.; Pitt, B.; Rossing, P.; Ruilope, L.; Gebel, M.; Kolkhof, P.; Nowack, C.; et al. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: The role of finerenone. Nephrol. Dial. Transplantat. 2022, 37, 1014–1023.
- Chaudhuri, A.; Ghanim, H.; Arora, P. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: A review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes. Metab. 2022, 24, 365–376.
- Lerma, E.; White, W.B.; Bakris, G. Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease. Postgrad. Med. 2022, 135, 224–233.
- Kintscher, U.; Bakris, G.L.; Kolkhof, P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br. J. Pharmacol. 2022, 179, 3220–3234.
- Barrera-Chimal, J.; Kolkhof, P.; Lima-Posada, I.; Joachim, A.; Rossignol, P.; Jaisser, F. Differentiation between emerging non-steroidal and established steroidal mineralocorticoid receptor antagonists: Head-to-head comparisons of pharmacological and clinical characteristics. Expert Opin. Investig. Drugs 2021, 30, 1141–1157.
- Pitt, B.; Filippatos, G.; Gheorghiade, M.; Kober, L.; Krum, H.; Ponikowski, P.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Rationale and design of ARTS: A randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur. J. Heart Fail. 2012, 14, 668–675.
- Bramlage, P.; Swift, S.L.; Thoenes, M.; Minguet, J.; Ferrero, C.; Schmieder, R.E. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur. J. Heart Fail. 2016, 18, 28–37.
- Amazit, L.; Billan, F.L.; Kolkhof, P.; Lamribet, K.; Viengchareun, S.; Fay, M.R.; Khan, J.A.; Hillisch, A.; Lombès, M.; Rafestin-Oblin, M.E.; et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J. Biol. Chem. 2015, 290, 21876–21889.
- Patel, V.; Joharapurkar, A.; Jain, M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev. Res. 2021, 82, 341–363.
- Tesch, G.H.; Young, M.J. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front. Pharmacol. 2017, 8, 313–321.
- Pandey, A.K.; Bhatt, D.L.; Cosentino, F.; Marx, N.; Rotstein, O.; Pitt, B.; Pandey, A.; Butler, J.; Verma, S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur. Heart J. 2022, 43, 2931–2945.
- Nishiyama, A.; Yao, L.; Nagai, Y.; Miyata, K.; Yoshizumi, M.; Kagami, S.; Kondo, S.; Kiyomoto, H.; Shokoji, T.; Kimura, S.; et al. Possible Contributions of Reactive Oxygen Species and Mitogen-Activated Protein Kinase to Renal Injury in Aldosterone/Salt-Induced Hypertensive Rats. Hypertension 2004, 43, 841–848.
- Mathew, J.T.; Patni, H.; Chaudhary, A.N.; Liang, W.; Gupta, A.; Chander, P.N.; Ding, G.; Singhal, P.C. Aldosterone induces mesangial cell apoptosis both in vivo and in vitro. Am. J. Physiol. Renal Physiol. 2008, 295, F73–F81.
- Diah, S.; Zhang, G.X.; Nagai, Y.; Zhang, W.; Gang, L.; Kimura, S.; Hamid, M.R.A.; Tamiya, T.; Nishiyama, A.; Hitomi, H. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp. Cell Res. 2008, 314, 3654–3662.
- Shibata, S.; Ishizawa, K.; Uchida, S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens. Res. 2017, 40, 221–225.
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508.
- Hashikabe, Y.; Suzuki, K.; Jojima, T.; Uchida, K.; Hattori, Y. Aldosterone impairs vascular endothelial cell function. J. Cardiovasc. Pharmacol. 2006, 47, 609–613.
- Iwashima, F.; Yoshimoto, T.; Minami, I.; Sakurada, M.; Hirono, Y.; Hirata, Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 2008, 149, 1009–1014.
- Jaffe, I.Z.; Tintut, Y.; Newfell, B.G.; Demer, L.L.; Mendelsohn, M.E. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 799–805.
- Barrera-Chimal, J.; Rocha, L.; Amador-Martínez, I.; Pérez-Villalva, R.; González, R.; Cortés-González, C.; Uribe, N.; Ramírez, V.; Berman, N.; Gamba, G.; et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol. Dial. Transplant. 2019, 34, 794–801.
- Schierke, F.; Wyrwoll, M.J.; Wisdorf, M.; Niedzielski, L.; Maase, M.; Ruck, T.; Meuth, S.G.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx contribute to Na+—Induced vascular inflammation. Sci. Rep. 2017, 7, 46476.
- Banki, N.F.; Ver, A.; Wagner, L.J.; Vannay, A.; Degrell, P.; Prokai, A.; Gellai, R.; Lenart, L.; Szakal, D.N.; Kenesei, E.; et al. Aldosterone antagonists in monotherapy are protective against streptozotocin-induced diabetic nephropathy in rats. PLoS ONE 2012, 7, e39938.
- Lian, M.; Hewitson, T.D.; Wigg, B.; Samuel, C.S.; Chow, F.; Becker, G.J. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol. Dial. Transplant. 2012, 27, 906–912.
- Lachaux, M.; Barrera-Chimal, J.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes. Metab. 2018, 20, 2399–2407.
- Kolkhof, P.; Bärfacker, L. Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 2017, 234, T125–T140.
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717.
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975.
- Savarese, G.; Carrero, J.J.; Pitt, B.; Anker, S.D.; Rosano, G.M.C.; Dahlström, U.; Lund, L.H. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: An analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2018, 20, 1326–1334.
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant hypertension: Detection, evaluation, and management a scientific statement from the American Heart Association. Hypertension 2018, 72, e53–e90.
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 2284–2309.
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229.
- Thomsen, R.W.; Nicolaisen, S.K.; Hasvold, P.; Sanchez, R.G.; Pedersen, L.; Adelborg, K.; Egstrup, K.; Egfjord, M.; Sørensen, H.T. Elevated potassium levels in patients with chronic kidney disease: Occurrence, risk factors and clinical outcomes—A Danish population-based cohort study. Nephrol. Dial. Transplant. 2018, 33, 1610–1620.
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70.
- Hene, R.J.; Boer, P.; Koomans, H.A.; Dorhout Mees, E.J. Plasma aldosterone concentrations in chronic renal disease. Kidney Int. 1982, 21, 98–101.
- Reams, G.P.; Bauer, J.H. Effect of Enalapril in Subjects with Hypertension Associated with Moderate to Severe Renal Dysfunction. Arch. Intern. Med. 1986, 146, 2145–2148.
- Bomback, A.S.; Klemmer, P.J. The incidence and implications of aldosterone breakthrough. Nat. Clin. Pract. Nephrol. 2007, 3, 486–492.
- Hou, J.; Xiong, W.; Cao, L.; Wen, X.; Li, A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin. Ther. 2015, 37, 2086–2103.
- Chung, E.Y.M.; Ruospo, M.; Natale, P.; Bolignano, D.; Navaneethan, S.D.; Palmer, S.C.; Strippoli, G.F.M. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD007004.
- Chen, Y.; Liu, P.; Chen, X.; Li, Y.; Zhang, F.; Wang, Y. Effects of Different Doses of Irbesartan Combined with Spironolactone on Urinary Albumin Excretion Rate in Elderly Patients with Early Type 2 Diabetic Nephropathy. Am. J. Med. Sci. 2018, 355, 418–424.
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): A prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312.
- Epstein, M.; Buckalew, V.; Altamirano, J.; Roniker, B.; Krause, S.; Kleiman, J. Eplerenone reduces proteinuria in type II diabetes mellitus: Implications for aldosterone involvement in the pathogenesis of renal dysfunction. J. Am. Coll. Cardiol. 2002, 39, 249.
- Le Billan, F.; Perrot, J.; Carceller, E.; Travers, S.; Viengchareun, S.; Kolkhof, P.; Lombès, M.; Fagart, J. Antagonistic effects of finerenone and spironolactone on the aldosterone-regulated transcriptome of human kidney cells. FASEB J. 2021, 35, e21314.
- Grune, J.; Beyhoff, N.; Smeir, E.; Chudek, R.; Blumrich, A.; Ban, Z.; Brix, S.; Betz, I.R.; Schupp, M.; Foryst-Ludwig, A.; et al. Selective Mineralocorticoid Receptor Cofactor Modulation as Molecular Basis for Finerenone’s Antifibrotic Activity. Hypertension 2018, 71, 599–608.
- Barrera-Chimal, J.; Estrela, G.R.; Lechner, S.M.; Giraud, S.; El Moghrabi, S.; Kaaki, S.; Kolkhof, P.; Hauet, T.; Jaisser, F. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018, 93, 1344–1355.
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463.
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy a randomized clinical trial. JAMA 2015, 314, 884–994.
- Filippatos, G.; Anker, S.D.; Böhm, M.; Gheorghiade, M.; Køber, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016, 37, 2105–2114.
- Kolkhof, P.; Nowack, C.; Eitner, F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr. Opin. Nephrol. Hypertens. 2015, 24, 417–424.
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263.
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.P.; Kolkhof, P.; Roberts, L.; et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients with Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152.
This entry is offline, you can click here to edit this entry!
