Plastic pollution in the world is widespread and growing. The environment is swamped with nanoplastics (<100 nm), and the health consequences of these less visible pollutants are unknown. Furthermore, there is evidence that microplastics can release nanoplastics by digestive disintegration, implying that macroplastic exposure can cause direct and indirect disease via nanoplastics. Nanoplastics enters an organism through the respiratory and gastro-intestinal tract where they accumulate into the liver through blood circulation via absorption, or epidermal infiltration. It is stated that macroplastics can cause damage directly at the site of exposure, whereas nanoplastics can influence the liver, causing subsequent damage to other organs. Multi-organ dysfunction is brought on by liver changes, and nanoplastics can readily enter the gut-liver axis and disturb the gut microflora.
- nanoplastics
- hepatic glucose metabolism
- lipid peroxidation
- metabolic dysfunction
- gut-liver axis
1. Introduction
2. Pathways Involved in Liver Metabolism
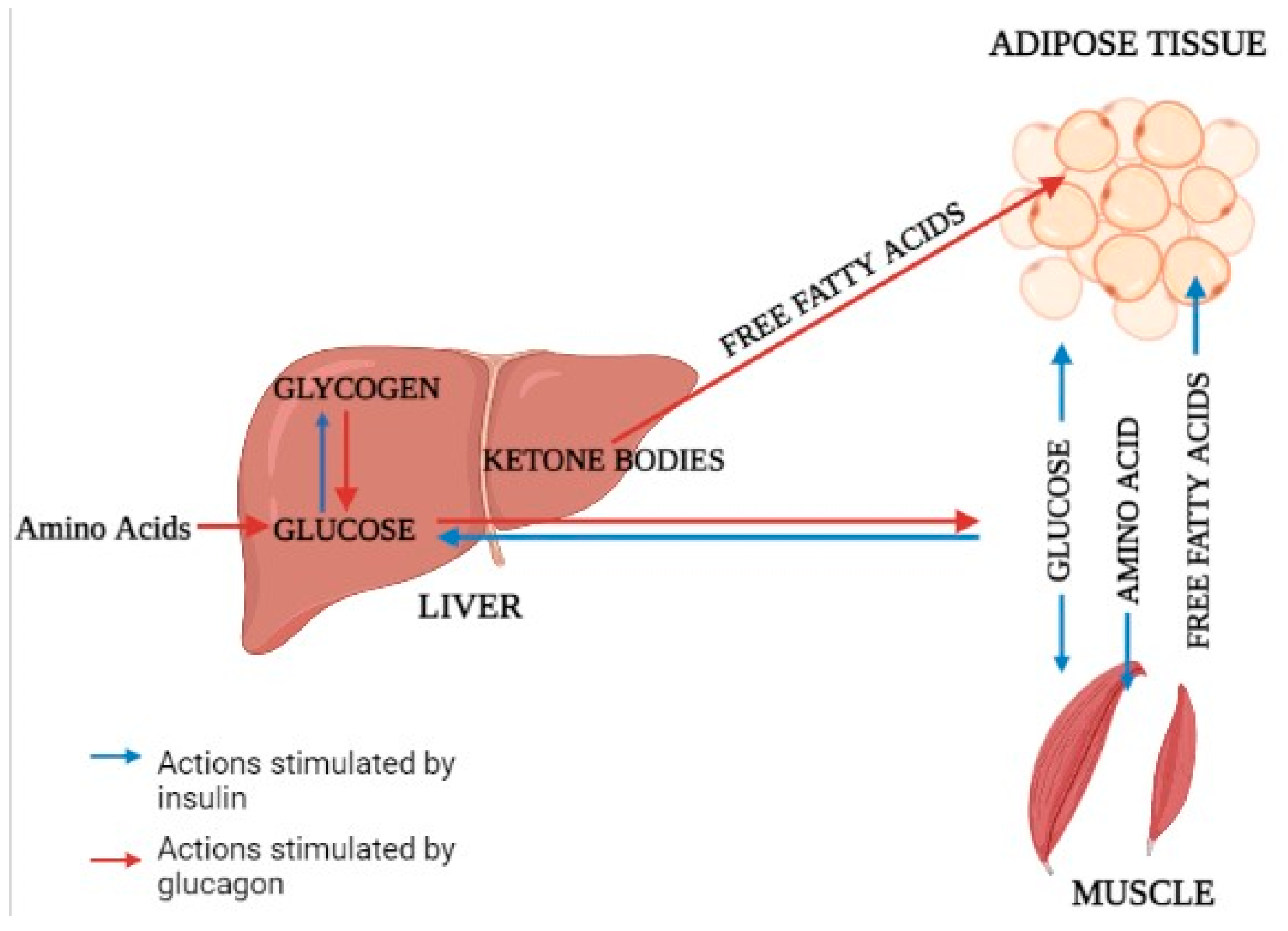
2.1. Glucose Metabolism
2.2. Glucose Output
2.3. Lipolysis and Lipid Oxidation
2.4. Fatty Acid Metabolism and Energy Supply
2.5. Mitochondrial Damage
2.6. Protein and Urea Metabolism
2.7. Ethanol Metabolism
3. Impact of Nanoplastics on Liver Metabolism Causing Multi-Organ Dysfunction
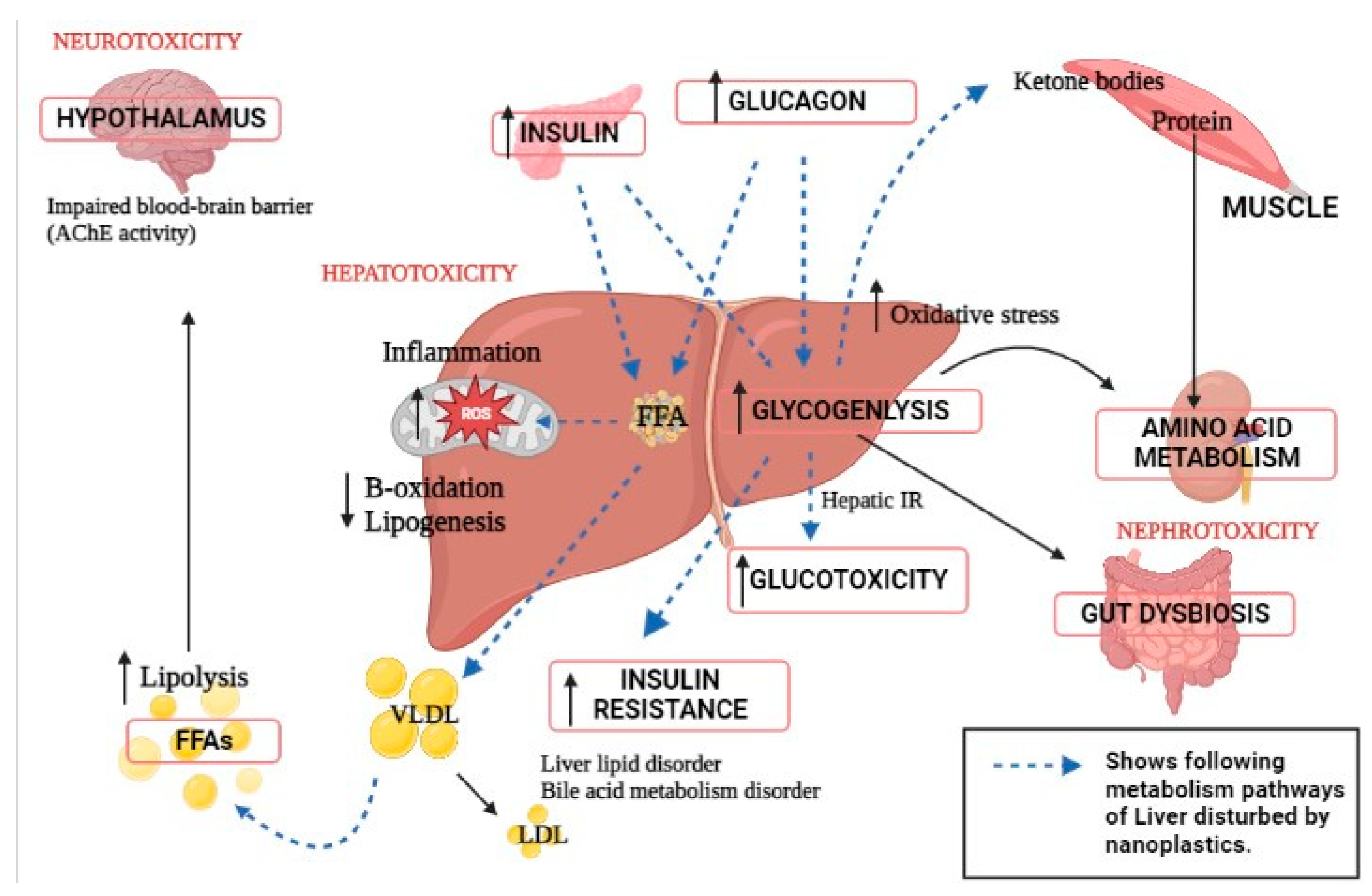
4. Alteration in Gut–Liver Axis Due to Nanoplastic Toxicity
This entry is adapted from the peer-reviewed paper 10.3390/genes14030590
References
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 1–13.
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32.
- Abbing, M.R. Plastic Soup: An Atlas of Ocean Pollution; Island Press: Washington DC, USA, 2019.
- Williams, M.; Gower, R.; Green, J.; Whitebread, E.; Lenkiewicz, Z.; Schröder, P. No Time to Waste: Tackling the Plastic Pollution Crisis before It’s Too Late; Tearfund: Teddington, UK, 2019.
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724.
- Li, Y.; Wang, Z.; Guan, B. Separation and Identification of Nanoplastics in Tap Water. Environ. Res. 2022, 204, 112134.
- Wang, H.; Shi, X.; Gao, Y.; Zhang, X.; Zhao, H.; Wang, L.; Zhang, X.; Chen, R. Polystyrene nanoplastics induce profound metabolic shift in human cells as revealed by integrated proteomic and metabolomic analysis. Environ. Int. 2022, 166, 107349.
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493.
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197.
- Lenard, N.R.; Berthoud, H.-R. Central and Peripheral Regulation of Food Intake and Physical Activity: Pathways and Genes. Obesity 2008, 16, S11–S22.
- Roseman, D.S.; Khan, T.; Rajas, F.; Jun, L.S.; Asrani, K.H.; Isaacs, C.; Farelli, J.D.; Subramanian, R.R. G6PC mRNA Therapy Positively Regulates Fasting Blood Glucose and Decreases Liver Abnormalities in a Mouse Model of Glycogen Storage Disease 1a. Mol. Ther. 2018, 26, 814–821.
- Hers, H.G.; Hue, L. Gluconeogenesis and related aspects of glycolysis. Annu. Rev. Biochem. 1983, 52, 617–653.
- Allen, M.S.; Bradford, B.J.; Oba, M. Board-invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334.
- Wei, Y.; Li, B.; Xu, H.; Liang, M. Liver Metabolome and Proteome Response of Turbot (Scophthalmus maximus) to Lysine and Leucine in Free and Dipeptide Forms. Front. Mar. Sci. 2021, 8, 691404.
- Kroupina, K.; Bémeur, C.; Rose, C.F. Amino acids, ammonia, and hepatic encephalopathy. Anal. Biochem. 2022, 649, 114696.
- Matsumoto, S.; Haberle, J.; Kido, J.; Mitsubuchi, H.; Endo, F.; Nakamura, K. Urea cycle disorders—Update. J. Hum. Genet. 2019, 64, 833–847.
- Campbell, I. Liver: Metabolic functions. Anaesth. Intensiv. Care Med. 2006, 7, 51–54.
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218.
- Wang, X.; Li, H.; Chen, Y.; Meng, X.; Dieketseng, M.Y.; Wang, X.; Yan, S.; Wang, B.; Zhou, L.; Zheng, G. A neglected risk of nanoplastics as revealed by the promoted transformation of plasmid-borne ampicillin resistance gene by Escherichia coli. Environ. Microbiol. 2022, 24, 4946–4959.
- Wang, H.; Li, Z.; Chen, H.; Jin, J.; Zhang, P.; Shen, L.; Hu, S.; Liu, H. Metabolomic analysis reveals the impact of ketoprofen on carbon and nitrogen metabolism in rice (Oryza sativa L.) seedling leaves. Environ. Sci. Pollut. Res. 2022, 30, 21825–21837.
- Ahmad, F.; Wang, X.; Li, W. Toxico-Metabolomics of Engineered Nanomaterials: Progress and Challenges. Adv. Funct. Mater. 2019, 29, 1904268.
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758.
- Xu, J.-L.; Lin, X.; Wang, J.J.; Gowen, A.A. A review of potential human health impacts of micro- and nanoplastics exposure. Sci. Total Environ. 2022, 851, 158111.
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ. 2020, 723, 138180.
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949.
- Li, X.; Wang, B.; Zhou, S.; Chen, W.; Chen, H.; Liang, S.; Zheng, L.; Yu, H.; Chu, R.; Wang, M.; et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J. Nanobiotechnology 2020, 18, 45.
- Xiao, J.; Jiang, X.; Zhou, Y.; Sumayyah, G.; Zhou, L.; Tu, B.; Qin, Q.; Qiu, J.; Qin, X.; Zou, Z.; et al. Results of a 30-day safety assessment in young mice orally exposed to polystyrene nanoparticles. Environ. Pollut. 2022, 292, 118184.
- Chi, Q.; Xu, T.; He, Y.; Li, Z.; Tang, X.; Fan, X.; Li, S. Polystyrene nanoparticle exposure supports ROS-NLRP3 axis-dependent DNA-NET to promote liver inflammation. J. Hazard. Mater. 2022, 439, 129502.
- Zhong, G.; Rao, G.; Tang, L.; Wu, S.; Tang, Z.; Huang, R.; Ruan, Z.; Hu, L. Combined effect of arsenic and polystyrene-nanoplastics at environmentally relevant concentrations in mice liver: Activation of apoptosis, pyroptosis and excessive autophagy. Chemosphere 2022, 300, 134566.
- Liao, H.; Liu, S.; Junaid, M.; Gao, D.; Ai, W.; Chen, G.; Wang, J. Di-(2-ethylhexyl) phthalate exacerbated the toxicity of polystyrene nanoplastics through histological damage and intestinal microbiota dysbiosis in freshwater Micropterus salmoides. Water Res. 2022, 219, 118608.
- Dimitriadis, G.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159.
- Gregory, J.M.; Kraft, G.; Scott, M.F.; Neal, D.W.; Farmer, B.; Smith, M.S.; Hastings, J.R.; Madsen, P.; Kjeldsen, T.B.; Hostrup, S.; et al. Peripherally delivered hepatopreferential insulin analog insulin-406 mimics the hypoglycaemia-sparing effect of portal vein human insulin infusion in dogs. Diabetes Obes. Metab. 2019, 21, 2294–2304.
- Williamson, G. Effects of Polyphenols on Glucose-Induced Metabolic Changes in Healthy Human Subjects and on Glucose Transporters. Mol. Nutr. Food Res. 2022, 66, 2101113.
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1273–1298.
- Lee, J.H.; Park, A.; Oh, K.-J.; Kim, W.K.; Bae, K.-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924.
- Sullivan, M.A.; Forbes, J.M. Glucose and glycogen in the diabetic kidney: Heroes or villains? Ebiomedicine 2019, 47, 590–597.
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Non-alcoholic fatty liver disease: An overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions. Life Sci. 2021, 271, 119220.
- Tas, E.; Bai, S.; Mak, D.; Diaz, E.C.; Dranoff, J.A. Obesity, but not glycemic control, predicts liver steatosis in children with type 1 diabetes. J. Diabetes Its Complicat. 2022, 36, 108341.
- Scapaticci, S.; D’Adamo, E.; Mohn, A.; Chiarelli, F.; Giannini, C. Non-Alcoholic Fatty Liver Disease in Obese Youth with Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021, 12, 639548.
- Kosmalski, M.; Ziółkowska, S.; Czarny, P.; Szemraj, J.; Pietras, T. The Coexistence of Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. J. Clin. Med. 2022, 11, 1375.
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4049.
- Guerra, S.; Gastaldelli, A. The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174.
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216.
- Zhang, X.; He, Z.; Si, Q.; Hu, X.; Yang, L.; Gu, X.; Du, L.; Wang, L.; Pan, L.; Li, Y.; et al. The Association of Sarcopenia and Visceral Obesity with Lean Nonalcoholic Fatty Liver Disease in Chinese Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2022, 2022, 2229139.
- Kwon, O. Glucose Metabolism. In Stroke Revisited: Diabetes in Stroke; Springer: Singapore, 2021; pp. 3–13.
- Bray, G.A.; Bouchard, C. The biology of human overfeeding: A systematic review. Obes. Rev. 2020, 21, e13040.
- Cline, G.W.; Naganawa, M.; Chen, L.; Chidsey, K.; Carvajal-Gonzalez, S.; Pawlak, S.; Rossulek, M.; Zhang, Y.; Bini, J.; McCarthy, T.J.; et al. Decreased VMAT2 in the pancreas of humans with type 2 diabetes mellitus measured in vivo by PET imaging. Diabetologia 2018, 61, 2598–2607.
- Asare-Bediako, I.; Paszkiewicz, R.L.; Kim, S.P.; Woolcott, O.O.; Kolka, C.M.; Burch, M.A.; Kabir, M.; Bergman, R.N. Variability of Directly Measured First-Pass Hepatic Insulin Extraction and Its Association With Insulin Sensitivity and Plasma Insulin. Diabetes 2018, 67, 1495–1503.
- Albrechtsen, N.J.W. The glucose-mobilizing effect of glucagon at fasting is mediated by cyclic AMP. Am. J. Physiol. Metab. 2021, 321, E571–E574.
- Clayton, R.P.; Herndon, D.N.; Abate, N.; Porter, C. The Effect of Burn Trauma on Lipid and Glucose Metabolism: Implications for Insulin Sensitivity. J. Burn Care Res. 2018, 39, 713–723.
- Axsom, J.E.; Schmidt, H.D.; Matura, L.A.; Libonati, J.R. The Influence of Epigenetic Modifications on Metabolic Changes in White Adipose Tissue and Liver and Their Potential Impact in Exercise. Front. Physiol. 2021, 12, 686270.
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223.
- Lim, X.Y. Characterisation of the First Specific Inhibitor of Ceramide Synthase 1. Ph.D. Doctoral Dissertation, University of New South Wales, Sydney, Australia, 2018.
- Kheder, M.H. The Role of the Equine Enteroinsular Axis in Insulin Dysregulation: In Vitro Mechanistic Insights for Disease Prevention. Doctoral Dissertation, Queensland University of Technology, Brisbane City, Australia, 2018.
- Tenen, D.G.; Chai, L.; Tan, J.L. Metabolic alterations and vulnerabilities in hepatocellular carcinoma. Gastroenterol. Rep. 2021, 9, 1–13.
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494.
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356.
- Lee, J.; Park, J.-S.; Roh, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharmacal Res. 2019, 42, 935–946.
- Tharyan, R.G. Transcription Factor nfyb-1 Regulates Mitochondrial Function and Promotes Longevity Induced by Mitochondrial Impairment. Doctoral Dissertation, Universität zu Köln, Köln, Germany, 2019.
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9.
- Sonti, S.; Tyagi, K.; Pande, A.; Daniel, R.; Sharma, A.L.; Tyagi, M. Crossroads of Drug Abuse and HIV Infection: Neurotoxicity and CNS Reservoir. Vaccines 2022, 10, 202.
- Di Ciaula, A.; Bonfrate, L.; Baj, J.; Khalil, M.; Garruti, G.; Stellaard, F.; Wang, H.H.; Wang, D.Q.-H.; Portincasa, P. Recent Advances in the Digestive, Metabolic and Therapeutic Effects of Farnesoid X Receptor and Fibroblast Growth Factor 19: From Cholesterol to Bile Acid Signaling. Nutrients 2022, 14, 4950.
- Holeček, M. The Role of Skeletal Muscle in The Pathogenesis of Altered Concentrations of Branched-Chain Amino Acids (Valine, Leucine, and Isoleucine) in Liver Cirrhosis, Diabetes, and Other Diseases. Physiol. Res. 2021, 70, 293–305.
- Warrillow, S.; Fisher, C.; Bellomo, R. Correction and control of hyperammonemia in acute liver failure: The impact of continuous renal replacement timing, intensity, and duration. Crit. Care Med. 2020, 48, 218–224.
- Sun, S.-X.; Wu, J.-L.; Lv, H.-B.; Zhang, H.-Y.; Zhang, J.; Limbu, S.M.; Qiao, F.; Chen, L.-Q.; Yang, Y.; Zhang, M.-L.; et al. Environmental estrogen exposure converts lipid metabolism in male fish to a female pattern mediated by AMPK and mTOR signaling pathways. J. Hazard. Mater. 2020, 394, 122537.
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060.
- Wang, Q.; He, G.; Mai, K. Modulation of lipid metabolism, immune parameters, and hepatic transferrin expression in juvenile turbot (Scophthalmus maximus L.) by increasing dietary linseed oil levels. Aquaculture 2016, 464, 489–496.
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861.
- Pedersen, A.F.; Meyer, D.N.; Petriv, A.-M.V.; Soto, A.L.; Shields, J.N.; Akemann, C.; Baker, B.B.; Tsou, W.-L.; Zhang, Y.; Baker, T.R. Nanoplastics impact the zebrafish (Danio rerio) transcriptome: Associated developmental and neurobehavioral consequences. Environ. Pollut. 2020, 266, 115090.
- Cedervall, T.; Hansson, L.A.; Lard, M.; Frohm, B.; Linse, S. Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish. PLoS ONE 2012, 7, e32254.
- Yan, J.; Liao, K.; Wang, T.; Mai, K.; Xu, W.; Ai, Q. Dietary Lipid Levels Influence Lipid Deposition in the Liver of Large Yellow Croaker (Larimichthys crocea) by Regulating Lipoprotein Receptors, Fatty Acid Uptake and Triacylglycerol Synthesis and Catabolism at the Transcriptional Level. PLoS ONE 2015, 10, e0129937.
- Lai, W.; Xu, D.; Li, J.; Wang, Z.; Ding, Y.; Wang, X.; Li, X.; Xu, N.; Mai, K.; Ai, Q. Dietary polystyrene nanoplastics exposure alters liver lipid metabolism and muscle nutritional quality in carnivorous marine fish large yellow croaker (Larimichthys crocea). J. Hazard. Mater. 2021, 419, 126454.
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem. Toxicol. 2022, 160, 112803.
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273.
- Yin, K.; Wang, D.; Zhang, Y.; Lu, H.; Wang, Y.; Xing, M. Dose-effect of polystyrene microplastics on digestive toxicity in chickens (Gallus gallus): Multi-omics reveals critical role of gut-liver axis. J. Adv. Res. 2022; (In Press).
