Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Clinical Neurology
DBS is an invasive but established neurosurgical procedure that involves the implantation of one or more electrodes into a targeted brain area, an implantable pulse generator (IPG), and an extension connecting the electrode to the IPG. The IPG contains the battery and circuitry, which generate the electrical signal that is delivered to the targeted brain structure. The DBS system allows for the delivery of electrical pulses to specific areas of the brain with minimal effects on nearby regions.
- neurosurgery
- neurology
- geriatrics
1. History of DBS
DBS is a relatively novel procedure, with most of the research conducted in the past 20 years. Prior to its development, surgical solutions were limited to ablation and the removal of affected brain regions, collectively known as craniotomy [18]. An example of such a procedure is thalamotomy, which entails the excision of the thalamus from the brain or sections of it [19]. While these procedures alleviated bradykinesia (slowness of movement) and dyskinesia (erratic excessive movement), they had major drawbacks including aphasia and dysphagia [20]. Even with the development of more precise surgical procedures such as stereo lesioning [21], the potential for adverse effects from the removal of brain tissue underscores the need for less invasive, better-tolerated therapies.
The use of DBS dates back to 1964, when Ohye et al. used a stereotactic procedure to stimulate the ventrolateral thalamic nucleus [22]. Since then, the volume of research on DBS has steadily increased (Figure 1). Initially, DBS was intended to treat PD and other movement disorders, and the FDA approved its use for PD and essential tremor in 1997 [23]. However, funding for DBS research declined following the development of levodopa in 1969, which may explain the lack of interest until the late 1990s [20]. The approval of thalamic DBS for the treatment of Parkinsonian symptoms in 1997 reignited interest in the field, and the potential applications of DBS in the treatment of other conditions such as AD, dystonia, epilepsy, and psychiatric disorders are currently being heavily researched [20]. As evident in Figure 1, while a significant amount of research has been conducted on the effectiveness of DBS treatment in PD, AD has received far less attention (Figure 1). Thus, further research on the potential use of DBS in AD is necessary.
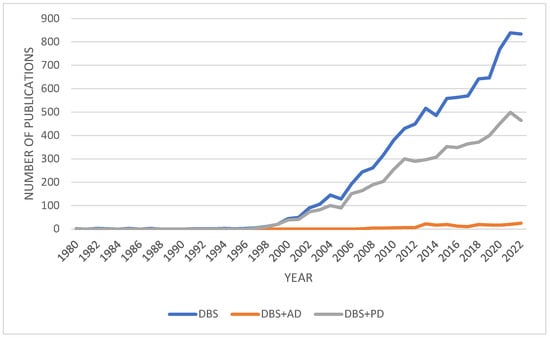
Figure 1. A graph of the number of research articles published on deep brain stimulation between 1980 and 2022. The queries performed were “DBS and deep brain stimulation”, “DBS and deep brain stimulation and AD and Alzheimer’s Disease”, and “DBS and deep brain stimulation and PD and Parkinson’s Disease”. Search parameters where limited to articles, books, and book chapters. Data extracted from Scopus: https://www.scopus.com (Accessed on 15 December 2022).
2. Current Treatment Plans
DBS can be used to modulate almost all regions of the brain for numerous neurologic conditions. The treatment process begins with the implantation of wires that taper into electrodes in the brain using a precise stereo lesioning procedure [16,21]. Different brain regions can be stimulated to alleviate the effects of different conditions (Table 1).
Table 1. A selection of conditions in which DBS is being actively researched as a viable treatment option.
| Condition | Area of Stimulation | First Author | Year of Publication | References |
|---|---|---|---|---|
| Parkinson’s Disease | Subthalamic nucleus (STN) | Deuschl, Xie | 2006, 2015 | [24,25] |
| Globus Pallidus internus (GPi) | Bloomstedt, Baker | 2018, 2010 | [26,27] | |
| Zona Incerta | Bloonstedt, Ossowska | 2018, 2020 | [26,28] | |
| Pedunculopontine nucleus | Thevathasan | 2018 | [29] | |
| Essential Tremor | Ventral intermediate thalamus | Baizabal-Carvallo | 2014 | [30] |
| Zona incerta | Fytagoridis | 2012 | [31] | |
| Dystonia | GPi | Vidailhet | 2013 | [32] |
| Alzheimer’s Disease | Fornix | [33,34] | ||
| Ventromedial prefrontal cortex | Mao, Lozano | 2018, 2016 | [35,36] | |
| Hippocampus | Scharre, Chakravarty | 2018, 2016 | [37] | |
| Nucleus Basalis Meynert | Kuhn | 2014 | [38] | |
| Huntington’s Disease | GPi | Velez-Lago | 2013 | [39] |
| Obsessive Compulsive Disorder (OCD) | Anteromedial GPi | Nair | 2014 | [40] |
| Nucleus Accumbens | Huff, Denys | 2010, 2010 | [41,42] | |
| Anterior limb of the internal capsule (ALIC) | Denys, Kammen | 2020, 2022 | [43,44] | |
| Ventral Capsule/Ventral Striatum (VC/VS) | Park, Kammen, Greenburg | 2019, 2022, 2008 | [44,45,46] | |
| STN | Kammen, Li, Chabardes | 2022,2020, 2013 | [44,47,48] | |
| Inferior thalamic peduncle | Germann, Lee, Kammen | 2022,2019, 2022 | [44,49,50] | |
| Bed nucleus of the stria terminalis | Luyten, Mosley, Raymaekers, Kammen | 2015, 2021, 2016, 2022 | [44,51,52,53] | |
| Epilepsy | Anterior nucleus of thalamus | Salanova | 2018 | [54] |
| Depression | VC/VS | Malone | 2009 | [55] |
| Nucleus Accumbens | Bewernick | 2010 | [56] |
Once the electrodes are positioned into the desired area of the brain, a separate implantable pulse generator (IPG) device is implanted in the chest wall with leads running under the skin [16]. The IPG can be adjusted externally to deliver a range of frequencies, pulse widths, and amplitudes to the targeted brain region unilaterally and bilaterally [20]. Different frequencies have been used to target specific symptoms, with high frequency stimulation (HFS) defined as above 100 Hz and low frequency stimulation (LFS) as 60–80 Hz [57,58,59]. However, this is quite subjective to each disease, as some papers cite that the range of 10–70 Hz is generally avoided due to the risk of eliciting seizures for epilepsy studies [59]. Some studies have found that frequencies around 60 Hz have restorative effects on PD symptoms, such as reducing aspiration and improving gait [25,60]. In particular, it is noted by di Blaise et al. that HFS systems decrease the levodopa-responsive PD symptoms, whereas LFS systems decrease more axial symptoms of the disease [58]. Indeed, Ramdhani et al. found that PD patients who experienced worsening of their symptoms after receiving HFS DBS had improvements in freezing of gait and dyskinesia when they were switched to a 60 Hz LFS system [60]. Contrastingly, Wyckhuys et al. discovered that HFS systems of 130 Hz are more effective at increasing the threshold and latency of after-discharges in kindled rats, a common animal model of epilepsy [59]. These studies suggest that the use of HFS and LFS systems is dependent on the type of disease that is to be treated, as well as the specific symptoms to be alleviated.
3. Patient Screening
Given the wide range of neurological disorders that can be treated by DBS, it is important to carefully select candidates for the treatment. One important factor to consider is the length of time since the patient’s diagnosis. There is conflicting evidence whether DBS is more effective for patients who were diagnosed within the past 5 years or for those who were diagnosed earlier. For PD, some studies suggest that earlier treatment may be more beneficial [61], while others suggest that it is important to monitor patients over a longer period of time to identify atypical symptoms and assess treatment suitability [62]. Moreover, surgically invasive procedures are usually left as a last-resort treatment and thus are usually used on older patients who have been diagnosed for longer. However, this approach benefits from the ability to rule out other “Parkinson-plus” diseases which may not be clinically treatable through DBS, such as comorbidities of PD and other diseases with similar symptoms [61].
Patients being considered for device-assisted therapies must undergo evaluation by a specialized center’s multi-disciplinary team, which typically includes a functional neurosurgeon, an anesthesiologist, a movement disorder neurologist, and a neuropsychologist, as well as representatives from departments such as radiology, psychiatry, physical therapy, nursing, and social work.
Another factor to consider when selecting candidates for DBS is the patient’s responsiveness to levodopa, a common medication used to treat PD. Many studies have found that patients who are responsive to levodopa are more likely to also respond to DBS [62,63], while some others have found conflicting evidence [64,65]. The site of stimulation may affect the relationship between levodopa responsiveness and DBS efficacy, but the reason for this is not well understood. Lin et al. found a positive correlation between levodopa responsiveness and GPi-DBS efficacy (R2 = 0.283, p = 0.016), though STN DBS was more efficacious than GPi-DBS for levodopa-resistant tremor control [66]. There is no current indication of how medication for AD may interact with DBS treatment, but co-therapy to mitigate the side effects is certainly something future clinical trials could investigate. In the future, online tools may be developed to help improve the selection process for DBS candidates and provide more uniformity in the selection process. One study found that the use of an online tool called Stimulus to screen 3128 patients led to a significantly higher acceptance rate than conventional multi-disciplinary screening [67].
4. Procedure and Mechanism of Action
The DBS hardware includes multiconductor intracranial quadripolar electrodes, a programmable single- or dual-channel internal pulse generator with battery unit, and an extension cable connecting the DBS electrodes to the pulse generator [20].
The procedure involves stereotactic placement of electrodes in the target area, either unilaterally or bilaterally (Figure 2). This is the first stage of the procedure and is often performed while the patient is awake. In the second stage, the electrode and extension cable are tunneled under the skin to the infraclavicular area, where they are connected to the battery-powered pulse generator [44].
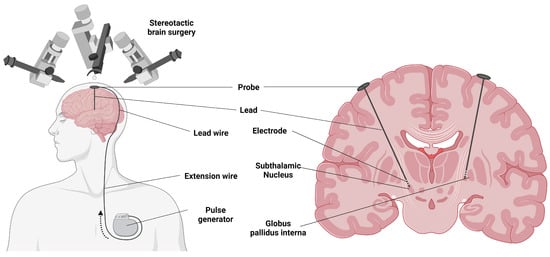
Figure 2. Electrode implantation for deep brain stimulation in Parkinson’s disease. Created using https://BioRender.com (Accessed on 19 December 2022).
When activated, the pulse generator delivers electric stimulation to the targeted area. The exact mechanism of action is not fully understood, but it is hypothesized that high-frequency DBS in PD suppresses GPi neuronal activity and efferent fiber pathways. This disrupts the flow of abnormal information through the cortico-basal ganglia circuits and downstream pathways, improving symptoms [68,69].
Overall, DBS as a procedure has come a long way since its initial development in the 1960s. Moreover, it is increasingly being trialed as an efficacious invasive procedure to alleviate the symptoms of many diseases. Additionally, more data are being generated on patient criteria which allows them to undergo ameliorative DBS treatment as well as the potential mechanisms by which DBS may affect different neurodegenerative diseases. Currently, the DBS only has regulatory approval in the treatment of PD.
This entry is adapted from the peer-reviewed paper 10.3390/cells12111478
This entry is offline, you can click here to edit this entry!
