Pathophysiological conditions such as endothelial dysfunction and arterial stiffness, characterized by low nitric oxide bioavailability, deficient endothelium-dependent vasodilation and heart effort, predispose individuals to atherosclerotic lesions and cardiac events. Nitrate (NO3−), L-arginine, L-citrulline and potassium (K+) can mitigate arterial dysfunction and stiffness by intensifying NO bioavailability. Dietary compounds such as L-arginine, L-citrulline, NO3− and K+ exert vasoactive effects as demonstrated in clinical interventions by noninvasive flow-mediated vasodilation (FMD) and pulse-wave velocity (PWV) prognostic techniques.
- L-arginine
- L-citrulline
- Potassium ions
- NO bioavailability
- dietary interventions
1. Introduction
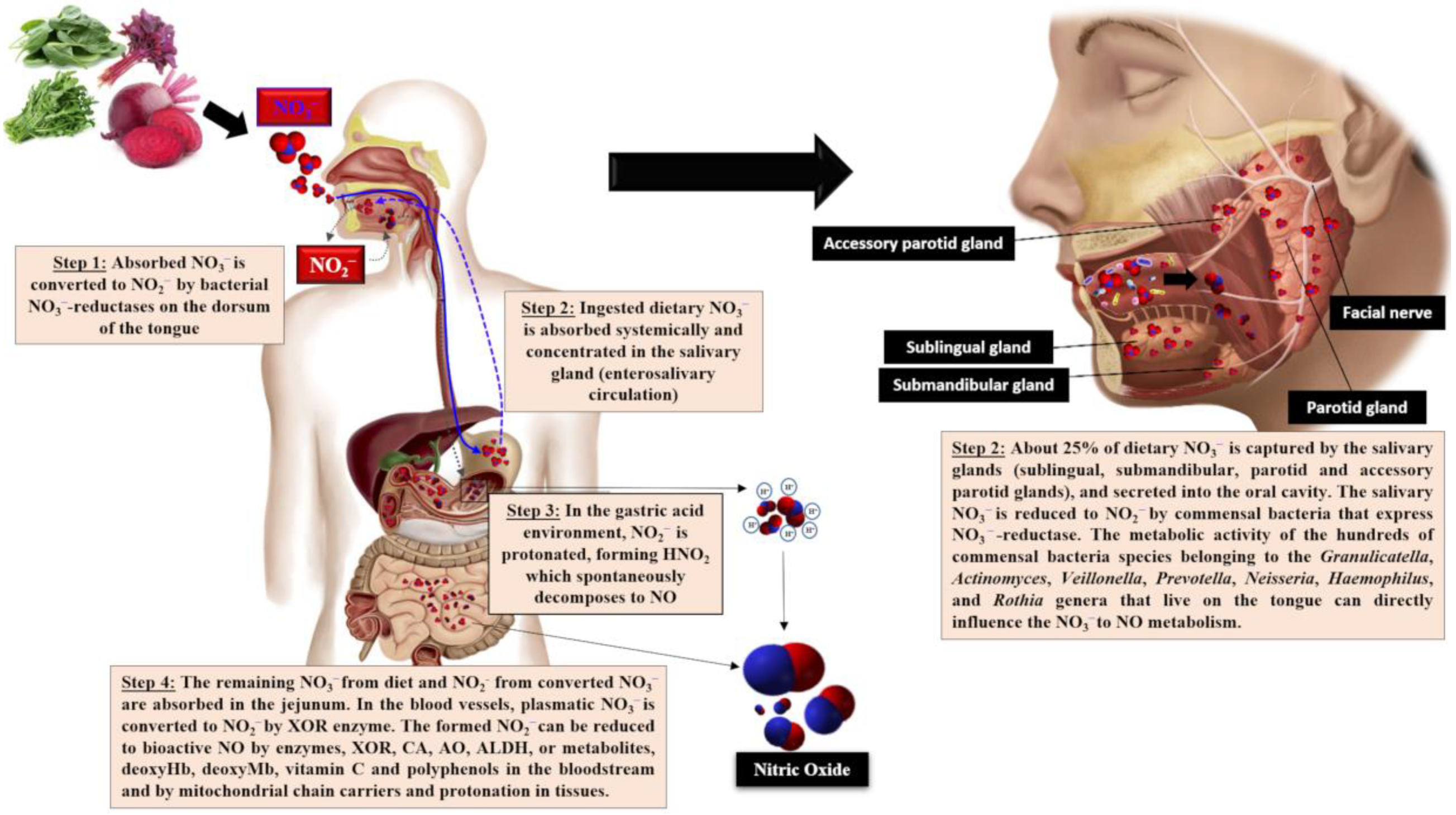
2. Nitrate
3. L-Arginine
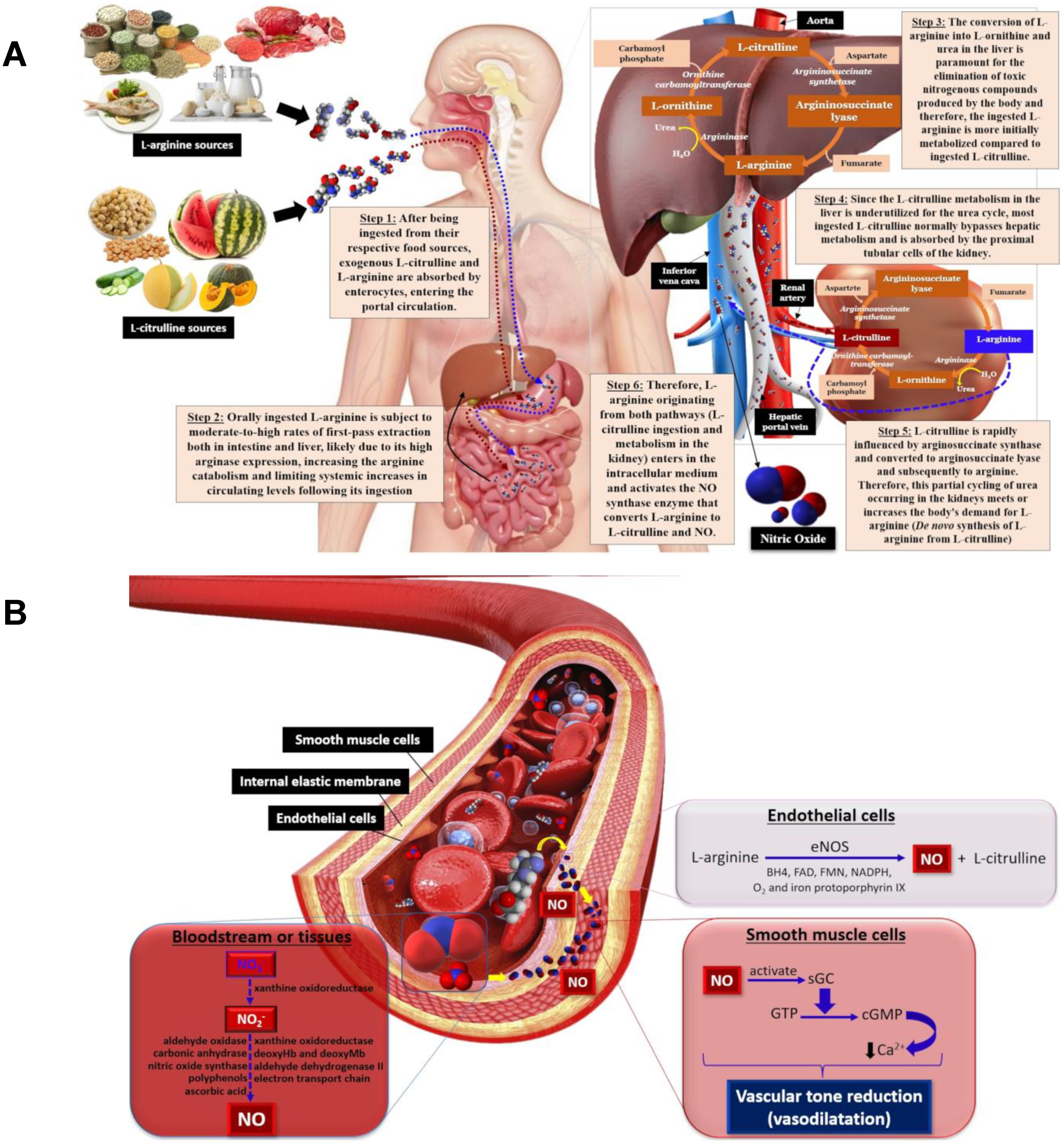
4. L-Citrulline
This entry is adapted from the peer-reviewed paper 10.3390/nu15112618
References
- Rahimi, N. Defenders and Challengers of Endothelial Barrier Function. Front. Immunol. 2017, 8, 1847.
- Sun, Y.; Liu, F.; Zhang, Y.; Lu, Y.; Su, Z.; Ji, H.; Cheng, Y.; Song, W.; Hidru, T.H.; Yang, X.; et al. The Relationship of Endothelial Function and Arterial Stiffness with Subclinical Target Organ Damage in Essential Hypertension. J. Clin. Hypertens. 2022, 24, 418–429.
- dos Santos Baião, D.; Vieira Teixeira da Silva, D.; Margaret Flosi Paschoalin, V. A Narrative Review on Dietary Strategies to Provide Nitric Oxide as a Non-Drug Cardiovascular Disease Therapy: Beetroot Formulations—A Smart Nutritional Intervention. Foods 2021, 10, 859.
- Lai, W.K.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12.
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of Free Fatty Acids in Endothelial Dysfunction. J. Biomed. 2017, 24, 50.
- Ohsugi, M.; Adachi, K.; Horai, R.; Kakuta, S.; Sudo, K.; Kotaki, H.; Tokai-Nishizumi, N.; Sagara, H.; Iwakura, Y.; Yamamoto, T. Kid-mediated Chromosome Compaction Ensures Proper Nuclear Envelope Formation. Cell 2008, 132, 771–782.
- Blumenthal, J.A.; Hinderliter, A.L.; Smith, P.J.; Mabe, S.; Watkins, L.L.; Craighead, L.; Ingle, K.; Tyson, C.; Lin, P.H.; Kraus, W.E.; et al. Effects of Lifestyle Modification on Patients with Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial. Circulation 2021, 144, 1212–1226.
- Ozkor, M.A.; Hayek, S.S.; Rahman, A.M.; Murrow, J.R.; Kavtaradze, N.; Lin, J.; Manatunga, A.; Quyyumi, A.A. Contribution of Endothelium-Derived Hyperpolarizing Factor to Exercise-induced Vasodilation in Health and Hypercholesterolemia. Vasc. Med. 2015, 20, 14–22.
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and Vascular Endothelial Function in Humans. Clin. Sci. 2011, 120, 357–375.
- Virdis, A. Endothelial Dysfunction in Obesity: Role of Inflammation. High Blood Press. Cardiovasc. Prev. 2016, 23, 83–85.
- Messner, B.; Bernhard, D. Smoking and Cardiovascular Disease: Mechanisms of Endothelial Dysfunction and Early Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515.
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annu. Rev. Pathol. 2009, 4, 71–95.
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619.
- Oliver, J.J.; Webb, D.J. Noninvasive Assessment of Arterial Stiffness and Risk of Atherosclerotic Events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 554–566.
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic Stiffness: Current Understanding and Future Directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522.
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943.
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, A. Age-Related Vascular Stiffening: Causes and Consequences. Front. Genet. 2015, 6, 112.
- Schram, M.T.; Schalkwijk, C.G.; Bootsma, H.A.; Fuller, J.H.; Chaturvedi, N.; Stehouwer, C.D.A. Advanced Glycation end Products are Associated with Pulse Pressure in Type 1 Diabetes. Hypertension 2005, 46, 232–237.
- Laurent, S.; Boutouyrie, P. The Structural Factor of Hypertension: Large and Small Artery Alterations. Circ. Res. 2015, 116, 1007–1021.
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH Oxidases and Oxidase Crosstalk in Cardiovascular Diseases: Novel Therapeutic Targets. Nat. Rev. Cardiol. 2020, 17, 170–194.
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1055–1062.
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to Measure Arterial Stiffness in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1034–1043.
- Tršan, J.; Košuta, D.; Rajkovič, U.; Fras, Z.; Jug, B.; Novaković, M. Vascular Function in Patients after Myocardial Infarction: The Importance of Physical Activity. Front. Physiol. 2021, 12, 763043.
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Endothelial Dysfunction, Increased Arterial Stiffness, and Cardiovascular Risk Prediction in Patients with Coronary Artery Disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J. Am. Heart Assoc. 2018, 7, e008588.
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567.
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuñiga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Marin, C.; Lopez-Moreno, J.; Castaño, J.P.; Delgado-Lista, J.; et al. Mediterranean Diet and Endothelial Function in Patients with Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. PLoS Med. 2020, 17, e1003282.
- Baião, D.D.S.; de Freitas, C.S.; Gomes, L.P.; da Silva, D.; Correa, A.C.N.T.F.; Pereira, P.R.; Aguila, E.M.D.; Paschoalin, V.M.F. Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases. Nutrients 2017, 9, 1044.
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular Disease Prevention by Diet Modification: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 914–926.
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Carlström, M.; Larsen, F.J.; Nyström, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary Inorganic Nitrate Reverses Features of Metabolic Syndrome in Endothelial Nitric Oxide Synthase-Deficient Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720.
- Zhao, Y.X.; Tong, L.; Zhang, G.M.; Zhao, X.H.; Sa, Y.P.; Liu, Y.; Lu, D.X.; Ga, Q.; Wu, P. L-Arginine Supplementation Improves Vascular Endothelial Dysfunction Induced by High-Fat Diet in Rats Exposed to Hypoxia. Wilderness Environ. Med. 2020, 31, 400–406.
- Tsuboi, T.; Maeda, M.; Hayashi, T. Administration of L-Arginine Plus L-Citrulline or L-Citrulline Alone Successfully Retarded Endothelial Senescence. PLoS ONE 2018, 13, e0192252.
- Zhou, M.S.; Kosaka, H.; Yoneyama, H. Potassium Augments Vascular Relaxation Mediated by Nitric Oxide in The Carotid Arteries of Hypertensive Dahl Rats. Am. J. Hypertens. 2000, 13, 666–672.
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-Arginine Improves Endothelial Function in Healthy Individuals Older than 70 Years. Vasc. Med. 2003, 8, 77–81.
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921.
- Raubenheimer, K.; Hickey, D.; Leveritt, M.; Fassett, R.; Ortiz de Zevallos Munoz, J.; Allen, J.D.; Briskey, D.; Parker, T.J.; Kerr, G.; Peake, J.M.; et al. Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients 2017, 22, 1270.
- Blanch, N.; Clifton, P.M.; Petersen, K.S.; Keogh, J.B. Effect of Sodium and Potassium Supplementation on Vascular and Endothelial Function: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 939–946.
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167.
- Nyawose, S.; Naidoo, R.; Naumovski, N.; McKune, A.J. The Effects of Consuming Amino Acids L-Arginine, L-Citrulline (and Their Combination) as a Beverage or Powder, on Athletic and Physical Performance: A Systematic Review. Beverages 2022, 8, 48.
- Nomura, N.; Shoda, W.; Uchida, S. Clinical Importance of Potassium Intake and Molecular Mechanism of Potassium Regulation. Clin. Exp. Nephrol. 2019, 23, 1175–1180.
- Ekmekcioglu, C.; Elmadfa, I.; Meyer, A.L.; Moeslinger, T. The Role of Dietary Potassium in Hypertension and Diabetes. J. Physiol. Biochem. 2016, 72, 93–106.
- Omar, S.A.; Artime, E.; Webb, A.J. A Comparison of Organic and Inorganic Nitrates/Nitrites. Nitric Oxide 2012, 26, 229–240.
- Alzahrani, H.S.; Jackson, K.G.; Hobbs, D.A.; Lovegrove, J.A. The Role of Dietary Nitrate and the Oral Microbiome on Blood Pressure and Vascular Tone. Nutr. Res. Rev. 2020, 34, 222–239.
- Reina-Torres, E.; De Leso, M.L.; Pasquale, L.R.; Madekurozwa, M.; van Batenburg-Sherwood, J.; Overby, D.R.; Daniel Stamer, W. The Vital Role for Nitric Oxide in Intraocular Pressure Homeostasis. Prog. Retin. Eye Res. 2021, 83, 100922.
- Gilchrist, M.; Winyard, P.G.; Benjamin, N. Dietary Nitrate—Good or Bad? Nitric Oxide 2010, 22, 104–109.
- Baião, D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, Bioactive and Physicochemical Characteristics of Different Beetroot Formulations. In Food Additives; Intech Open: London, UK, 2017; Volume 2, pp. 20–44.
- González-Soltero, R.; Bailén, M.; de Lucas, B.; Ramírez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611.
- Lidder, S.; Webb, A.J. Vascular Effects of Dietary Nitrate (as Found in Green Leafy Vegetables and Beetroot) via the Nitrate-Nitrite-Nitric Oxide Pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696.
- Blekkenhorst, L.C.; Bondonno, N.P.; Liu, A.H.; Ward, N.C.; Prince, R.L.; Lewis, J.R.; Devine, A.; Croft, K.D.; Hodgson, J.M.; Bondonno, C.P. Nitrate, the Oral Microbiome, and Cardiovascular Health: A Systematic Literature Review of Human and Animal Studies. Am. J. Clin. Nutr. 2018, 107, 504–522.
- Burleigh, M.; Liddle, L.; Muggeridge, D.J.; Monaghan, C.; Sculthorpe, N.; Butcher, J.; Henriquez, F.; Easton, C. Dietary Nitrate Supplementation Alters the Oral Microbiome but Does Not Improve the Vascular Responses to an Acute Nitrate Dose. Nitric Oxide 2019, 89, 54–63.
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241.
- McDonagh, S.T.J.; Wylie, L.J.; Webster, J.M.A.; Vanhatalo, A.; Jones, A.M. Influence of Dietary Nitrate Food Forms on Nitrate Metabolism and Blood Pressure in Healthy Normotensive Adults. Nitric Oxide 2018, 72, 66–74.
- Weitzberg, E.; Lundberg, J.O. Novel Aspects of Dietary Nitrate and Human Health. Annu. Rev. Nutr. 2013, 33, 129–159.
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to Increase Nitric Oxide Signalling in Cardiovascular Disease. Nat. Rev. Drug Discov. 2015, 14, 623–641.
- Woessner, M.N.; Levinger, I.; Neil, C.; Smith, C.; Allen, J.D. Effects of Dietary Inorganic Nitrate Supplementation on Exercise Performance in Patients with Heart Failure: Protocol for a Randomized, Placebo-Controlled, Cross-Over Trial. JMIR Res. Protoc. 2018, 7, e86.
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017, 105, 48–67.
- Tamme, T.; Reinik, M.; Püssa, T.; Roasto, M.; Meremäe, K.; Kiis, A. Dynamics of Nitrate and Nitrite Content During Storage of Home-Made and Small-Scale Industrially Produced Raw Vegetable Juices and Their Dietary Intake. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 487–495.
- Baião, D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can Be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960.
- Spiegelhalder, B.; Eisenbrand, G.; Preussman, R. Influence of Dietary Nitrate on Nitrite Content of Human Saliva: Possible Relevance to In-Vivo Formation of N-Nitroso Compounds. Food Cosmet. Toxicol. 1976, 14, 545–548.
- Tannenbaum, S.R.; Weisman, M.; Fett, D. The Effect of Nitrate Intake on Nitrite Formation in Human Saliva. Food Cosmet. Toxicol. 1976, 14, 549–552.
- Powlson, D.S.; Addiscott, T.M.; Benjamin, N.; Cassman, K.G.; de Kok, T.M.; van Grinsven, H.; L’Hirondel, J.L.; Avery, A.A.; van Kessel, C. When Does Nitrate Become a Risk For Humans? J. Environ. Qual. 2008, 37, 291–295.
- Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO). Evaluation of Certain Food Additives and Contaminants; WHO: Geneva, Switzerland, 1995; Available online: https://apps.who.int/iris/bitstream/handle/10665/42849/WHO_TRS_922.pdf;sequence=1 (accessed on 30 January 2023).
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the European Commission to Perform a Scientific Risk Assessment on Nitrate in Vegetables. EFSA J. 2008, 6, 689. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/689 (accessed on 30 January 2023).
- Katan, M.B. Nitrate in Foods: Harmful or Healthy? Am. J. Clin. Nutr. 2009, 90, 11–12.
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O., III; et al. Nitrite Infusion in Humans and Nonhuman Primates: Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation 2007, 116, 1821–1831.
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food Sources of Nitrates and Nitrites: The Physiologic Context for Potential Health Benefits. Am. J. Clin. Nutr. 2009, 90, 1–10.
- Chazelas, E.; Pierre, F.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaesse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; Srour, B.; et al. Nitrites and Nitrates from Food Additives and Natural Sources and Cancer Risk: Results from the Nutrinet-Santé Cohort. Int. J. Epidemiol. 2022, 51, 1106–1119.
- Van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The Oral Bioavailability of Nitrate from Nitrate-Rich Vegetables in Humans. Toxicol. Lett. 2008, 181, 177–181.
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of Dietary Nitrate on Blood Pressure, Endothelial Function, and Insulin Sensitivity in Type 2 Diabetes. Free Radic. Biol. Med. 2013, 60, 89–97.
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Yang, X.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-Term Effects of Nitrate-Rich Green Leafy Vegetables on Blood Pressure and Arterial Stiffness in Individuals with High-Normal Blood Pressure. Free Radic. Biol. Med. 2014, 77, 353–362.
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-Term Effects of a High Nitrate Diet on Nitrate Metabolism in Healthy Individuals. Nutrients 2015, 7, 1906–1915.
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients: A Randomized, Phase 2, Double-Blind, Placebo Controlled Study. Hypertension 2015, 65, 320–327.
- Rammos, C.; Hendgen-Cotta, U.B.; Sobierajski, J.; Bernard, A.; Kelm, M.; Rassaf, T. Dietary Nitrate Reverses Vascular Dysfunction in Older Adults with Moderately Increased Cardiovascular Risk. J. Am. Coll. Cardiol 2014, 63, 1584–1585.
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The Nitrate-Independent Blood Pressure-Lowering Effect of Beetroot Juice: A Systematic Review and Meta-Analysis. Hypertension 2015, 65, 320–327.
- Lara, J.; Ashor, A.W.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C.; Siervo, M. Effects of Inorganic Nitrate and Beetroot Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2016, 55, 451–459.
- Wijnands, K.A.; Meesters, D.M.; van Barneveld, K.W.; Visschers, R.G.; Briedé, J.J.; Vandendriessche, B.; Van Eijk, H.M.; Bessems, B.A.; van den Hoven, N.; Von Wintersdorff, C.J.; et al. Citrulline Supplementation Improves Organ Perfusion and Arginine Availability Under Conditions with Enhanced Arginase Activity. Nutrients 2015, 7, 5217–5238.
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615.
- Ataabadi, A.E.; Golshiri, K.; Jüttner, A.; Krenning, G.; Danser, A.H.J.; Roks, A.J. Nitric Oxide-cGMP Signaling in Hypertension: Current and Future Options for Pharmacotherapy. Hypertension 2020, 76, 1055–1068.
- Blatter, L.A.; Wier, W.G. Nitric Oxide Decreases in Vascular Smooth Muscle by Inhibition of the Calcium Current. Cell Calcium 1994, 15, 122–131.
- Vtolix, M.L.; Raeymaeken, F.; Wuytack, F.; Hofmann, F.; Casteels, R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem. J. 1988, 255, 855–863.
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005, 25, 1551–1557.
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E. Variation in L-Arginine Intake Follow Demographics and Lifestyle Factors That May Impact Cardiovascular Disease Risk. Nutr. Res. 2008, 28, 21–24.
- Siasos, G.; Tousoulis, D.; Vlachopoulos, C.; Antoniades, C.; Stefanadi, E.; Ioakeimidis, N.; Andreou, I.; Zisimos, K.; Papavassiliou, A.G.; Stefanadis, C. Short-Term Treatment with L-Arginine Prevents the Smoking-Induced Impairment of Endothelial Function and Vascular Elastic Properties in Young Individuals. Int. J. Cardiol. 2008, 126, 394–399.
- Siasos, G.; Tousoulis, D.; Vlachopoulos, C.; Antoniades, C.; Stefanadi, E.; Ioakeimidis, N.; Zisimos, K.; Siasou, Z.; Papavassiliou, A.G.; Stefanadis, C. The impact of oral L-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am. J. Hypertens. 2009, 22, 586–592.
- Adams, M.R.; McCredie, R.; Jessup, W.; Robinson, J.; Sullivan, D.; Celermajer, D.S. Oral L-Arginine Improves Endothelium-Dependent Dilatation and Reduces Monocyte Adhesion to Endothelial Cells in Young Men with Coronary Artery Disease. Atherosclerosis 1997, 129, 261–269.
- Yin, W.H.; Chen, J.W.; Tsai, C.; Chiang, M.C.; Young, M.S.; Lin, S.J. L-Arginine Improves Endothelial Function and Reduces LDL Oxidation in Patients with Stable Coronary Artery Disease. Clin. Nutr. 2005, 24, 988–997.
- Lucotti, P.; Monti, L.; Setola, E.; La Canna, G.; Castiglioni, A.; Rossodivita, A.; Pala, M.G.; Formica, F.; Paolini, G.; Catapano, A.L.; et al. Oral L-Arginine Supplementation Improves Endothelial Function and Ameliorates Insulin Sensitivity and Inflammation in Cardiopathic Nondiabetic Patients after an Aortocoronary Bypass. Metabolism 2009, 58, 1270–1276.
- Schächinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic Impact of Coronary Vasodilator Dysfunction on Adverse Long-Term Outcome of Coronary Heart Disease. Circulation 2000, 101, 1899–1906.
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic Ovary Syndrome and Cardiometabolic Risk: Opportunities for Cardiovascular Disease Prevention. Trends Cardiovasc. Med. 2020, 30, 399–404.
- Battaglia, C.; Mancini, F.; Battaglia, B.; Facchinetti, F.; Artini, P.G.; Venturoli, S. L-Arginine Plus Drospirenone-Ethinyl Estradiol in the Treatment of Patients with PCOS: A Prospective, Placebo Controlled, Randomized, Pilot Study. Gynecol. Endocrinol. 2010, 26, 861–868.
- Orea-Tejeda, A.; Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Keirns-Davies, C.; Montano-Hernández, P.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Infante, O.; Martínez-Memije, R. The Effect of L-Arginine and Citrulline on Endothelial Function in Patients in Heart Failure with Preserved Ejection Fraction. Cardiol. J. 2010, 17, 464–470.
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008, 117, 2467–2474.
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172.
- Annavarajula, S.K.; Dakshinamurty, K.V.; Naidu, M.U.; Reddy, C.P. The Effect of L-Arginine on Arterial Stiffness and Oxidative Stress in Chronic Kidney Disease. Indian J. Nephrol. 2012, 22, 340–346.
- Gaenzer, H.; Sturm, W.; Neumayr, G.; Kirchmair, R.; Ebenbichler, C.; Ritsch, A.; Föger, B.; Weiss, G.; Patsch, J.R. Pronounced postprandial lipemia impairs endothelium-dependent dilation of the brachial artery in men. Cardiovasc. Res. 2001, 52, 509–516.
- Ceriello, A.; Taboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, B.; Da Ros, R.; Motz, E. Evidence for an Independent and Cumulative Effect of Postprandial Hypertriglyceridemia and Hyperglycemia on Endothelial Dysfunction and Oxidative Stress Generation—Effects of Short- and Long-Term Simvastatin Treatment. Circulation 2002, 106, 1211–1218.
- Fewkes, J.J.; Kellow, N.J.; Cowan, S.F.; Williamson, G.; Dordevic, A.L. A single, high-fat meal adversely affects postprandial endothelial function: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2022, 116, 699–729.
- Deveaux, A.; Pham, I.; West, S.G.; André, E.; Lantoine-Adam, F.; Bunouf, P.; Sadi, S.; Hermier, D.; Mathé, V.; Fouillet, H.; et al. L-Arginine Supplementation Alleviates Postprandial Endothelial Dysfunction When Baseline Fasting Plasma Arginine Concentration is Low: A Randomized Controlled Trial in Healthy Overweight Adults with Cardiometabolic Risk Factors. J. Nutr. 2016, 146, 1330–1340.
- Lin, C.C.; Tsai, W.C.; Chen, J.Y.; Li, Y.H.; Lin, L.J.; Chen, J.H. Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int. J. Cardiol. 2008, 127, 337–341.
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290.
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293.
- Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Narváez-David, R.; Keirns-Davis, C.; Carrasco-Ortiz, O.; Navarro-Navarro, A.; Sánchez-Santillán, R. Effect of L-arginine or L-Citrulline Oral Supplementation on Blood Pressure and Right Ventricular Function in Heart Failure Patients with Preserved Ejection Fraction. Cardiol. J. 2010, 17, 612–618.
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of Watermelon Supplementation on Aortic Blood Pressure and Wave Reflection in Individuals with Prehypertension: A Pilot Study. Am. J. Hypertens. 2011, 24, 40–44.
- Akashi, K.; Mifune, Y.; Morita, K.; Ishitsuka, S.; Tsujimoto, H.; Ishihara, T. Spatial Accumulation Pattern of Citrulline and Other Nutrients in Immature and Mature Watermelon Fruits. J. Sci. Food Agric. 2017, 97, 479–487.
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive Compounds from Flesh and By-Product of Fresh-Cut Watermelon Cultivars. J. Sci. Food Agric. 2011, 91, 805–812.
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two Weeks of Watermelon Juice Supplementation Improves Nitric Oxide Bioavailability but Not Endurance Exercise Performance in Humans. Nitric Oxide-Biol. Chem. 2016, 59, 10–20.
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-Citrulline and Watermelon Supplementation on Vascular Function and Exercise Performance. Curr. Opin. Clin. Nutr. Metab. 2017, 20, 92–98.
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From Metabolism to Therapeutic Use. Nutrition 2013, 29, 479–484.
- Fan, J.; Park, E.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.M.; Sandhu, A.K. Pharmacokinetic Parameters of Watermelon (Rind, Flesh and Seeds) Bioactive Components in Human Plasma: A Pilot Study to Investigate the Relationship to Endothelial Function. J. Agric. Food Chem. 2020, 68, 7393–7403.
- Volino-Souza, M.; de Oliveira, G.V.; Conte-Junior, C.A.; Figueroa, A.; Alvares, S.T. Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective. Nutrients 2022, 14, 2913.
- Vincellette, C.M.; Losso, J.; Early, K.; Spielmann, G.; Irving, B.A.; Allerton, T.D. Supplemental Watermelon Juice Attenuates Acute Hyperglycemia-Induced Macro-and Microvascular Dysfunction in Healthy Adults. J. Nutr. 2021, 151, 3450–3458.
- Ellis, A.C.; Mehta, T.; Nagabooshanam, V.A.; Dudenbostel, T.; Locher, J.L.; Crowe-White, K.M. Daily 100% Watermelon Juice Consumption and Vascular Function among Postmenopausal Women: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 2959–2968.
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects Of Watermelon Supplementation on Arterial Stiffness and Wave Reflection Amplitude in Postmenopausal Women. Menopause 2013, 20, 573–577.
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon Extract Supplementation Reduces Ankle Blood Pressure and Carotid Augmentation Index in Obese Adults with Prehypertension or Hypertension. Am. J. Hypertens. 2012, 25, 640–643.
- Kim, I.Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.; Wolfe, R.R. Acute Ingestion of Citrulline Stimulates Nitric Oxide Synthesis but Does Not Increase Blood Flow in Healthy Young and Older Adults with Heart Failure. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E915–E924.
