Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Wu Jianming.
Megakaryocytes (MKs), a kind of functional hematopoietic stem cell, form platelets to maintain platelet balance through cell differentiation and maturation. The incidence of blood diseases such as thrombocytopenia has increased, but these diseases cannot be fundamentally solved.
- botany
- blood system
- research progress
- megakaryocyte
1. Introduction
Platelets are well known for their powerful roles in vascular injury, inflammation and wound healing [1]. However, megakaryocytes, the precursor cells of platelets, have not been well known until recent decades. Megakaryocytes, a type of hematopoietic cell, undergo a series of complex processes to generate platelets, such as growth, development and maturation. At the same time, megakaryocytes possess certain cellular capabilities of immunity and inflammation [2]. In recent years, various experiments have mutually confirmed that megakaryocytes play an important role in the process of self-renewal and differentiation of HSC into multifunctional lineages [3,4,5][3][4][5]. In addition, as a type of intercellular communication cells, megakaryocytes also maintain the function of niches in the bone marrow [6]. Therefore, megakaryocytes play a very important role in the hematopoietic system.
Megakaryocytes release an average of 1 × 1011 platelets into the blood each day. Megakaryocyte differentiation and maturation and platelet production are complex processes regulated by strict factors. This process is a hot topic in the field of hematopoiesis. In most clinical cases, thrombocytopenia is defined as a platelet count less than 100 × 109/L, with clinical manifestations of splenomegaly, purpura, skin and mucous membrane hemorrhage, visceral hemorrhage, intracranial hemorrhage and even death [7]. Thrombocytopenia is a secondary complication of many diseases, such as myelodysplastic syndrome [8], leukemia [9], dengue fever [7], and parasitic infections [10]. In addition, myelosuppression caused by chemoradiotherapy also presents with a decreased platelet count [11]. To treat thrombocytopenia, platelet infusion, cytokines and TPO receptor agonists are commonly used in clinic [12]. However, platelet supply is scarce, the cost is high, the storage time of platelet is short, and it is easily contaminated by bacteria. Bloodborne infections may exist in the transfusion process, and homologous immune-related complications may also be caused. Cytokines stimulate platelet production by increasing the number of hematopoietic stem cells and blood cells. However, cytokines have many adverse reactions, including arrhythmia and acute pulmonary edema [13]. Thrombopoietin receptor agonists currently include first-generation recombinant human thrombopoietin (RhTPO) and second-generation agents (TPO-RAs), such as romiplostim and eltrombopag. Although they promote megakaryocyte differentiation and platelet production in vivo and increase the number of peripheral blood platelets, RhTPO may cross-react with endogenous TPO of patients to neutralize autoantibodies, which may result in immunogenic reactions, while romiplostim is the only TPO-RA that binds with endogenous TPO at the same site without resulting in an immunogenic reaction. However, there is a risk of thrombosis and up to 10% discontinue reaction. Both them require regular treatment and frequent monitoring. [14,15,16][14][15][16]. In recent years, ideas of platelet generation in vitro have been proposed, and many laboratories have successfully differentiated megakaryocytes into platelets in vitro. However, the large-scale production of platelets is still a challenging task in vitro [17].
Although the above treatment agents have significant efficacy, they have many adverse reactions and are limited in clinical application.
2. The Process of Megakaryocyte Differentiation
Megakaryocyte differentiation is a complex process in which hematopoietic stem cells differentiate into megakaryocytes, undergo terminal differentiation, and finally form proplatelets. In the classical process of hematopoietic stem cell self-renewal and directional differentiation, long-term hematopoietic stem cells differentiate into multiple multifunctional progenitor cells, then multiple multifunctional progenitor cells differentiate into myeloid progenitors and lymphoid progenitors, myeloid progenitors differentiate into megakaryocyte-erythroid progenitors and granulocyte-macrophage progenitors, and eventually differentiate into megakaryocyte progenitors and erythroid precursors [19][18]. Megakaryocyte progenitors eventually differentiate into megakaryocytes. After terminal differentiation and maturation, megakaryocytes eventually form proplatelets [20][19]. However, several recent studies have found that in addition to the ability to self-renew, long-term hematopoietic stem cells (LT-HSCs) can differentiate into short-term hematopoietic stem cells (ST-HSCs), which can differentiate into multiple multifunctional progenitors [21][20]. They are mainly divided into MPP1, MPP2, MPP3 and MPP4 (Figure 1). MPP1 is classified as a short-term HSC. MPP3 is largely granulocyte/macrophage-biased. MPP4 differentiates into common lymphoid progenitor cells (CLPs), which differentiate into T cells and B cells. MPP2 [22][21] cells are progenitor cells that tend to differentiate into common myeloid progenitors (CMPs). CMPs can differentiate into granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythroid progenitors (MEPs). MEPs differentiate into erythroid progenitors (EryPs) and megakaryocytic progenitors (MkPs). At the same time, MPP2 cells could skip the process from HSCs to CMPs and MEPs and directly differentiate into megakaryocytes. Another study reported that approximately 60% of hematopoietic stem cells are VWF+ cells in the bone marrow. In the hematopoietic microenvironment, because megakaryocytes generate platelets, they exist in vascular sinuses, and VWF + HSC have the same transcription factors and cytokines. Megakaryocytes are thought to be predisposed to induce VWF + HSC to differentiate into megakaryocytes and generate platelets [3]. Therefore, wresearchers hypothesize that VWF+ cells have a specific tendency to differentiate into myeloid lineage cells under certain conditions. These two pathways can improve the efficiency of megakaryocytes differentiation in the hematopoietic lineage and provide an effective method for rapid platelet production in the hematopoietic system. After that, megakaryocytes will undergo multiple mitoses to undergo polyploid terminal differentiation and maturation, in which the largest polyploid nucleus reaches 128 N. Once megakaryocytes mature, they extend proplatelets into the vascular sinuses through the energy provided by the cytoskeleton and then release platelets through shear forces [23][22].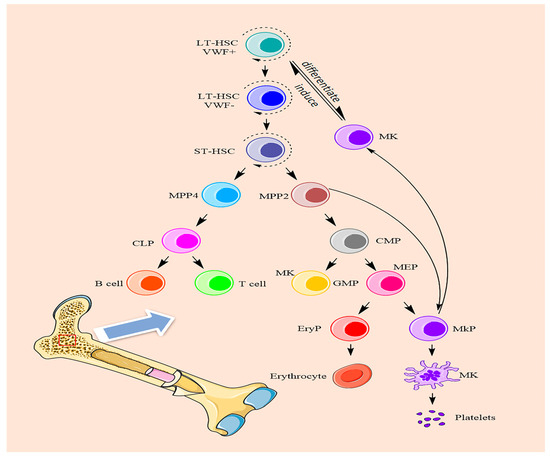
Figure 1. The differentiation processes of megakaryocytes.
3. Molecular Mechanisms Affecting Megakaryocyte Differentiation
3.1. The Role of Signaling Pathways in Megakaryocyte Differentiation
Megakaryocytes are regulated by transcription factors, adhesion factors, cytokines and chemokines in the hematopoietic system. Among these mediators, thrombopoietin (TPO), the ligand for the c-MPL receptor, is the most important for megakaryocyte generation and differentiation (Figure 2A). TPO is considered to be a key regulator of megakaryocyte differentiation and can initiate a variety of signal transduction pathways, including JAK2/STAT3/STAT5, MAPK/ERK and PI3K/AKT [24,25][23][24]. ERK1/2 promotes the differentiation of HSCs into megakaryocytes under continuous activation [26][25]. PI3K/AKT has been implicated in the late differentiation of megakaryocytes [27][26]. As a downstream signaling pathway of JAK2, STAT3/STAT5 is also involved in megakaryocyte maturation and platelet generation. Some researchers knocked out the c-MPL receptor in mice and found that the mice still had a small number of platelets left in their blood that kept them alive. However, there is no evidence that TPO is involved in the late differentiation of megakaryocytes and platelet formation.
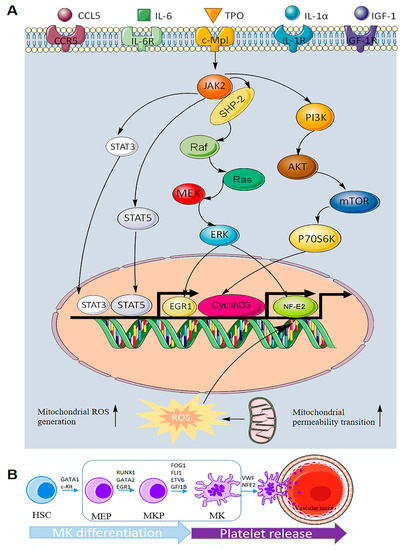
Figure 2. Molecular mechanism of megakaryocyte differentiation. (A) The role of signaling pathways in megakaryocyte differentiation. There are at least five regulatory factors in megakaryocyte differentiation, the most important of which is TPO, which mainly initiates three signaling pathways, namely, JAK2/STAT3/STAT5, MAPK/ERK and PI3K/AKT. These intracellular factors related to pathway regulation can promote megakaryocyte differentiation and the generation of platelets. In addition, increased mitochondrial permeability and ROS can also promote megakaryocyte differentiation. (B) Transcription factors that play a role in each stage of MK differentiation and platelet release.
Therefore, some scholars have made a reasonable guess that the differentiation and maturation of megakaryocytes are regulated by a variety of other factors apart from TPO. Interleukins, such as IL-6, IL-11 and IL-1α, can stimulate megakaryocyte differentiation and promote platelet formation. Vascular endothelial growth factor (VEGF) is secreted by various hematopoietic and nonhematopoietic cells and has three homologous receptor tyrosine kinases (VEGFR1/VEGFR2/VEGFR3) that play roles in different stages of megakaryocyte differentiation [28][27]. Insulin growth factor (IGF-1) can promote CD34+ cells to differentiate into megakaryocytes and promote platelet formation [29][28]. Tyrosyl-tRNA synthetase (YRSACT) was shown to induce the differentiation of hematopoietic stem cells in TPO-deficient signaling [30][29]. Chemokine ligand 5 (CCL5, RANTES) has been shown to bind to CCR5, a receptor of megakaryocytes, to promote megakaryocyte differentiation and proplatelet generation [31][30]. In addition, human growth hormone (hGH) may exert a complementary and synergistic effect with c-MPL ligands on thrombopoiesis [32][31]. The discovery of these factors has gradually completed the process of megakaryocyte differentiation in the hematopoietic system, which provides the possibility for the drug treatment of megakaryocyte-related diseases.
3.2. The Role of Transcription Factors in Megakaryocyte Differentiation
Megakaryocyte differentiation depends largely on the expression of transcription factors in the nucleus. Various transcription factors play an important role in each stage of HSC differentiation into megakaryocytes (Figure 2B), such as GATA1, FOG1, FLI1, RUNX1, NFE2 and SCL2. GATA1 affects the differentiation of HSCs into megakaryocyte-erythroid progenitors [33][32]. GATA2 promotes MEP differentiation into megakaryocytes accompanied by erythropoiesis. RUNX1, a master regulator of the hematopoietic system, inhibits the erythroid-specific transcription factor KLF1, thereby changing the ratio of FIL1/KLF1 and promoting megakaryocyte differentiation. RUNX1 may also promote hematopoietic lineage migration to megakaryocytes by downregulating the expression of MYH10, a gene encoding non-muscle myosin heavy chains [4]. Early growth factor (EGR1) can promote MEP differentiation into megakaryocytes [2]. Other transcription factors, such as FOG1, FLI1, ETV6 and GFI1B, can promote megakaryocyte differentiation [34][33]. VWF+ and NF-E2 also promote the differentiation and maturation of megakaryocytes to generate proplatelets.
Transcription factors play an integral role in megakaryocyte differentiation and platelet release, ensuring a stable number of circulating platelets under stable conditions. However, there are still a large number of transcription factors involved in the complex regulatory process of megakaryocyte differentiation that have not been fully explained.
References
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1288–1300.
- Crist, S.A.; Elzey, B.D.; Ahmann, M.T.; Ratliff, T.L. Early growth response-1 (EGR-1) and nuclear factor of activated T cells (NFAT) cooperate to mediate CD40L expression in megakaryocytes and platelets. J. Biol. Chem. 2013, 288, 33985–33996.
- Sanjuan-Pla, A.; Macaulay, I.C.; Jensen, C.T.; Woll, P.S.; Luis, T.C.; Mead, A.; Moore, S.; Carella, C.; Matsuoka, S.; Bouriez Jones, T.; et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 2013, 502, 232–236.
- Eto, K.; Kunishima, S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood 2016, 127, 1234–1241.
- Notta, F.; Zandi, S.; Takayama, N.; Dobson, S.; Gan, O.I.; Wilson, G.; Kaufmann, K.B.; McLeod, J.; Laurenti, E.; Dunant, C.F.; et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 2016, 351, aab2116.
- Grinenko, T.; Arndt, K.; Portz, M.; Mende, N.; Gunther, M.; Cosgun, K.N.; Alexopoulou, D.; Lakshmanaperumal, N.; Henry, I.; Dahl, A.; et al. Clonal expansion capacity defines two consecutive developmental stages of long-term hematopoietic stem cells. J. Exp. Med. 2014, 211, 209–215.
- Lien, T.S.; Chan, H.; Sun, D.S.; Wu, J.C.; Lin, Y.Y.; Lin, G.L.; Chang, H.H. Exposure of platelets to dengue virus and envelope protein domain iii induces nlrp3 inflammasome-dependent platelet cell death and thrombocytopenia in mice. Front. Immunol. 2021, 12, 616394.
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 1399–1420.
- Repsold, L.; Pool, R.; Karodia, M.; Tintinger, G.; Joubert, A.M. An overview of the role of platelets in angiogenesis, apoptosis and autophagy in chronic myeloid leukaemia. Cancer Cell Int. 2017, 17, 89.
- Zhu, M.; Rong, X.; Li, M.; Wang, S. IL-18 and IL-35 in the serum of patients with sepsis thrombocytopenia and the clinical significance. Exp. Ther. Med. 2020, 19, 1251–1258.
- Yang, S.; Che, H.; Xiao, L.; Zhao, B.; Liu, S. Traditional Chinese medicine on treating myelosuppression after chemotherapy: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e24307.
- Bylsma, L.C.; Fryzek, J.P.; Cetin, K.; Callaghan, F.; Bezold, C.; Mehta, B.; Wasser, J.S. Systematic literature review of treatments used for adult immune thrombocytopenia in the second-line setting. Am. J. Hematol. 2019, 94, 118–132.
- Nguyen, A.; Repesse, Y.; Ebbo, M.; Allenbach, Y.; Benveniste, O.; Vallat, J.M.; Magy, L.; Deshayes, S.; Maigne, G.; de Boysson, H.; et al. IVIg increases interleukin-11 levels, which in turn contribute to increased platelets, VWF and FVIII in mice and humans. Clin. Exp. Immunol. 2021, 204, 258–266.
- Ghanima, W.; Cooper, N.; Rodeghiero, F.; Godeau, B.; Bussel, J.B. Thrombopoietin receptor agonists: Ten years later. Haematologica 2019, 104, 1112–1123.
- Gilreath, J.; Lo, M.; Bubalo, J. Thrombopoietin receptor agonists (tpo-ras): Drug class considerations for pharmacists. Drugs 2021, 81, 1285–1305.
- Al-Samkari, H.; Soff, G.A. Clinical challenges and promising therapies for chemotherapy-induced thrombocytopenia. Expert Rev. Hematol. 2021, 14, 437–448.
- Martinez-Botia, P.; Acebes-Huerta, A.; Seghatchian, J.; Gutierrez, L. On the Quest for In vitro platelet production by re-tailoring the concepts of megakaryocyte differentiation. Medicina 2020, 56, 671.
- Tatsutoshi, N.; Nobuo, O. Cell surface antigen expression in human erythroid progenitors: Erythroid and megakaryocytic markers. Leuk. Lymphoma 1994, 13, 401–409.
- Psaila, B.; Mead, A.J. Single-cell approaches reveal novel cellular pathways for megakaryocyte and erythroid differentiation. Blood 2019, 133, 1427–1435.
- Cabezas-Wallscheid, N.; Klimmeck, D.; Hansson, J.; Lipka, D.B.; Reyes, A.; Wang, Q.; Weichenhan, D.; Lier, A.; von Paleske, L.; Renders, S.; et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell 2014, 15, 507–522.
- Pietras, E.M.; Reynaud, D.; Kang, Y.A.; Carlin, D.; Calero-Nieto, F.J.; Leavitt, A.D.; Stuart, J.M.; Gottgens, B.; Passegue, E. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 2015, 17, 35–46.
- Bender, M.; Thon, J.N.; Ehrlicher, A.J.; Wu, S.; Mazutis, L.; Deschmann, E.; Sola-Visner, M.; Italiano, J.E.; Hartwig, J.H. Microtubule sliding drives proplatelet elongation and is dependent on cytoplasmic dynein. Blood 2015, 125, 860–868.
- Ng, A.P.; Kauppi, M.; Metcalf, D.; Hyland, C.D.; Josefsson, E.C.; Lebois, M.; Zhang, J.G.; Baldwin, T.M.; Di Rago, L.; Hilton, D.J.; et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc. Natl. Acad. Sci. USA 2014, 111, 5884–5889.
- Wang, L.; Zhang, T.; Liu, S.; Mo, Q.; Jiang, N.; Chen, Q.; Yang, J.; Han, Y.W.; Chen, J.P.; Huang, F.H.; et al. Discovery of a novel megakaryopoiesis enhancer, ingenol, promoting thrombopoiesis through PI3K-Akt signaling independent of thrombopoietin. Pharmacol. Res. 2022, 177, 106096.
- Kawaguchi, T.; Hatano, R.; Yamaguchi, K.; Nawa, K.; Hashimoto, R.; Yokota, H. Fibronectin promotes proplatelet formation in the human megakaryocytic cell line UT-7/TPO. Cell Biol. Int. 2012, 36, 39–45.
- Guerriero, R.; Parolini, I.; Testa, U.; Samoggia, P.; Petrucci, E.; Sargiacomo, M.; Chelucci, C.; Gabbianelli, M.; Peschle, C. Expression of Concern: Inhibition of TPO-induced MEK or mTOR activity induces opposite effects on the ploidy of human differentiating megakaryocytes. J. Cell Sci. 2019, 132, 744–752.
- Behrens, K.; Alexander, W.S. Cytokine control of megakaryopoiesis. Growth Factors 2018, 36, 89–103.
- Chen, S.; Hu, M.; Shen, M.; Wang, S.; Wang, C.; Chen, F.; Tang, Y.; Wang, X.; Zeng, H.; Chen, M.; et al. IGF-1 facilitates thrombopoiesis primarily through Akt activation. Blood 2018, 132, 210–222.
- Kanaji, T.; Vo, M.N.; Kanaji, S.; Zarpellon, A.; Shapiro, R.; Morodomi, Y.; Yuzuriha, A.; Eto, K.; Belani, R.; Do, M.H.; et al. Tyrosyl-tRNA synthetase stimulates thrombopoietin-independent hematopoiesis accelerating recovery from thrombocytopenia. Proc. Natl. Acad. Sci. USA 2018, 115, E8228–E8235.
- Machlus, K.R.; Johnson, K.E.; Kulenthirarajan, R.; Forward, J.A.; Tippy, M.D.; Soussou, T.S.; El-Husayni, S.H.; Wu, S.K.; Wang, S.; Watnick, R.S.; et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood 2016, 127, 921–926.
- Xu, Y.; Wang, S.; Shen, M.; Zhang, Z.; Chen, S.; Chen, F.; Chen, M.; Zeng, D.; Wang, A.; Zhao, J.; et al. hGH promotes megakaryocyte differentiation and exerts a complementary effect with c-Mpl ligands on thrombopoiesis. Blood 2014, 123, 2250–2260.
- Muntean, A.G.; Pang, L.; Poncz, M.; Dowdy, S.F.; Blobel, G.A.; Crispino, J.D. Cyclin D-Cdk4 is regulated by GATA-1 and required for megakaryocyte growth and polyploidization. Blood 2007, 109, 5199–5207.
- Takayama, M.; Fujita, R.; Suzuki, M.; Okuyama, R.; Aiba, S.; Motohashi, H.; Yamamoto, M. Genetic analysis of hierarchical regulation for Gata1 and NF-E2 p45 gene expression in megakaryopoiesis. Mol. Cell Biol. 2010, 30, 2668–2680.
More
