Hydrogen is an important green energy source and chemical raw material for various industrial processes. The major technique of hydrogen production is steam methane reforming (SMR), which suffers from high energy penalties and enormous CO2 emissions. As an alternative, chemical looping water-splitting (CLWS) technology represents an energy-efficient and environmentally friendly method for hydrogen production. The key to CLWS lies in the selection of suitable oxygen carriers (OCs) that hold outstanding sintering resistance, structural reversibility, and capability to release lattice oxygen and deoxygenate the steam for hydrogen generation.
- chemical looping
- water-splitting
- hydrogen
- oxygen carrier
1. Introduction
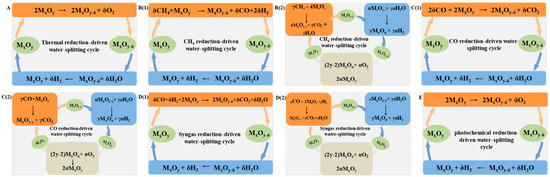
2. Processes for Chemical Looping Water-Splitting
2.1. Two-Step Thermochemical Water-Splitting
2.2. Methane Chemical Looping Process
The two-step TCWS cycle approach has substantially decreased the reaction temperature (≤1500 °C) compared to direct thermal splitting of water, but the high reaction temperature still puts forward tough requirements for the reaction equipment. Furthermore, the continuous temperature swing and thermal shock during the cyclic reaction not only reduces the reaction efficiency but also leads to rapid decrease of redox performance, which seriously limits the commercialization of this technology. To this end, recent reports verified that introduction of reducing gas could significantly accelerate the reduction kinetics of OCs, lower the reaction temperature to below 1000 °C, and increase the amount of lattice oxygen desorption, substantially enhancing the hydrogen production efficiency [37][38][39][40][41][79,80,81,82,83]. Methane, the main component of natural gas, has been widely studied due to its abundant reserves, high hydrogen-to-carbon ratio, and strong reducibility [42][43][84,85]. The two-step TCWS cycle with methane as reducing gas is commonly referred to as methane chemical looping process (Figure 12B). The facile reduction of OCs in methane atmosphere enables a closed reaction loop at much milder conditions, which potentially reduces the energy penalty and improves the economics of this process. Compared with conventional standalone systems that generate hydrogen and electricity separately based on methane, it is assessed that chemical looping hydrogen generation with methane as reduction gas was able to save more than 16% of energy input while reducing beyond 98% of CO2 emissions, rendering a low cost of $32.87/MWh for H2 production [44][86]. The methane conversion over OCs undergoes different processes, including partial oxidation or total combustion, when modulating the redox properties of these oxides. As for OCs with relatively low reducibility, syngas production is favored by selective methane oxidation (CH4 + OL → CO + 2 H2) (Figure 12B1). The reduced OCs can be subsequently recovered by water oxidation with generation of hydrogen (H2O → OL + H2). The overall reaction is generally referred as chemical looping steam methane reforming (CL-SMR) [45][87]. Compared with traditional steam methane reforming reaction (CH4 + H2O → CO + 3 H2), the CL-SMR process can obtain syngas with H2/CO ratio of 2, which is suitable for Fischer–Tropsch synthesis and methanol.2.2.1. Supported or Doped Iron Oxides
As for methane-driven three step CLWS, the studied OCs are mainly focused on iron oxides, which holds the virtue of low cost and environmentally friendly features [46][47][93,94]. However, developing an efficient method to avoid coke formation, which can degrade the hydrogen purity in the water-splitting step, over these OCs during methane atmosphere remains a great challenge [48][95]. Ku et al. [49][96] reported that methane dissociation and coke formation occurred over Fe2O3/Al2O3 OCs due to the generation of the FeAl2O4 phase, since the newly formed phase displayed poor oxygen mobility and rendered notably decreased oxygen capacity of OC for chemical looping reactions. To address this issue, Xiang et al. [50][97] proposed that loading Fe2O3 to MgAl2O4 support greatly suppressed the solid-phase reaction between iron oxides and the support, thereby restraining the carbon formation from CH4 dissociation. Furthermore, they found that K-promoted Fe2O3/Al2O3 further improved the resistance towards coke deposition by decreasing the reduction activity of oxygen carrier. Li et al. [51][98] found that a combination of mixed ionic-electronic conductive (MIEC) support of La0.8Sr0.2FeO3 with Fe2O3 improved lattice oxygen diffusion from the bulk to surface, which promoted the elimination of carbon during the methane reduction process.2.2.2. Supported or Doped Cerium Oxides
Cerium-based OCs have been widely studied for CL-SMR in terms of their high oxygen storage capacity and reliable resistance to carbon deposition [52][53][54][105,106,107]. However, pure CeO2 exhibits low reactivity towards methane conversion and is prone to sintering in several cyclic reactions. Therefore, great effort has been applied to improve the reactivity and stability of these OCs by doping foreign cations and constructing composite oxides. Wang et al. [55][108] discovered that Ni-modified (5 wt%) CeO2-TiO2 enabled 100% conversion of CH4, ca. 16 times greater than that of pure CeO2-TiO2 (5.9%), with 85% syngas selectivity at 900 °C. A mechanism study showed that Ni species accounted for the main active centers for CH4 activation and promoted the reduction of Ce4+ (CeO2-TiO2) to Ce3+ (Ce2Ti2O7). The synergy between active Ni species and CeO2-TiO2/Ce2Ti2O7 redox oxides significantly increased the water-splitting performance with hydrogen yield up to 47.0 mL/g.2.2.3. Perovskites
Perovskite oxides are promising OCs for CL-SMR due to their excellent redox activity [56][57][115,116]. Lee et al. [58][117] discovered that doping Fe in B-site of LaCoO3 improved lattice oxygen mobility, reactivity for partial oxidation of CH4, and adsorption and dissociation of H2O, which enabled high CO selectivity of 92% and H2 purity of 99.3% during the methane reduction and water-splitting steps, respectively. Gong and colleagues [59][60][118,119] discovered that substitution of Ce3+ for La3+ of LaFeO3 was capable of tuning lattice oxygen activity via modulating the distortion degree of FeO6 octahedra. When Ce/La ratio reached 1 (La0.5Ce0.5FeO3), the oxygen carrier had the lowest oxygen vacancy formation energy and the highest oxygen mobility, which rendered the best methane-to-syngas and water-splitting performance. Furthermore, the yield of hydrogen (0.6 mmol/g) remained stable during 100 redox cycles, highlighting the outstanding stability of this OC.2.3. Chemical Looping Water Gas Shift Process
As a low-cost but important platform chemical, CO can be used as a reductant-like methane to drive the CLWS process and produce high-value hydrogen (Figure 12C) [61][62][127,128]. Herein, OCs were reduced by CO (CO + OL → CO2) and subsequently regenerated by water (H2O → OL + H2). This process is generally known as a chemical looping water gas shift (CL-WGS) reaction due to the same overall reaction as that of water–gas shift reaction (CO + H2O → CO2 + H2). Compared with CL-SMR, the application of CO as a reducing atmosphere can avoid the carbon deposition caused by CH4 decomposition while the coke formation from the Boudouard reaction (2CO → C + CO2) is thermodynamically constrained at a temperature above 800 °C, which inhibited the contamination of hydrogen by carbon monoxide (C + H2O → CO + H2). Furthermore, the separation of CO/CO2 from H2O/H2 in chemical looping reactions also prevents the reverse water–gas shift reaction (CO2 + H2 → CO + H2O), leading to increased efficiency for water-splitting. Thermodynamic analysis demonstrated that the steam conversion in CL-WGS reaction reached 95% at 800 °C, which is significantly higher than the traditional WGS process [21][32]. As for CL-WGS, the suitable OCs should be reactive for CO oxidation and subsequent water-splitting while possessing high redox stability. Fe2O3 has been widely studied due to the features of abundant reserves, favorable thermodynamic properties, and high oxygen-carrying capacity [63][130]. However, Fe2O3 is prone to agglomerate during several redox cycles, rendering rapid decline of hydrogen productivity. Recent works showed that loading of Fe2O3 on some inert supports, including ZrO2 [64][131], Al2O3 [65][66][132,133], and MgAl2O4 [67][134], were effective in improving the specific surface area of OCs and slowing down the sintering process. Iron spinels that bear features of low cost, high thermal stability, and good oxygen mobility are promising OCs for chemical looping reactions [68][69][140,141]. Huang et al. [70][142] found that NiFe2O4/Al2O3 displayed stable H2 yield during 20 cycles of CL-WGS, since Al2O3 support significantly inhibited the sintering of NiFe2O4. Kim et al. [71][143] also found that Al-modified NiFe2O4 exhibited good performance for water-splitting due to the formation of a spinel solid solution with Al, which could prevent densification of NiFe2O4. For the optimized sample with Al content of 3.3 wt%, maximum hydrogen productivity of 8.2 mmol/g was reached. Cui et al. [72][144] reported that the active Fe could completely exsolve from mixed Zn-Fe-Al spinel in CO atmosphere and resolved into spinel structure by water oxidation, enabling a hydrogen yield of 2.23 mmol/g with nearly 100% conversion of CO. Brownmillerite (Ca2Fe2O5) is well known for high oxygen capacity and excellent thermal stability due to the anion-deficient structure with alternating FeO6 octahedral and FeO4 tetrahedral layers [73][74][147,148]. Based on the thermodynamic study, Ismail et al. [75][149] found that the valence state of Fe in Ca2Fe2O5 only displayed Fe0 and Fe3+ without intermediate Fe2+ during the redox reactions, and Fe0 could be directly oxidized to Fe3+ by steam, which was suitable for a water-splitting reaction. Chan et al. [76][150] discovered that the steam conversion over Ca2Fe2O5 could reach 75%, which was much higher than that of Fe2O3/ZrO2 (62%). Through thermogravimetric experiments, Sun et al. [77][151] found that Ca2Fe2O5 displayed much faster reaction kinetics than CaFe2O4 during the CO reduction step, rendering a higher reduction degree of 94.0% (85.5% for CaFe2O4).2.4. Syngas Chemical Looping Process
Syngas that can be produced via various routes, e.g., gasification of biomass, coal, and methane reforming, and represents another promising reducing agent for CLWS [78][79][80][153,154,155]. In the syngas-promoted CLWS process (Figure 12D), referred to as syngas chemical looping (SCL), syngas was utilized as the reducing gas instead of CO, while subsequent water-splitting and air oxidation (in some cases) were needed to recover the OCs. The focused OCs explored for this process are mainly iron-based oxides. Fan et al. [22][33] investigated the feasibility of Ni, Cu, Cd, Co, Mn, Sn, and Fe oxides for SCL through thermodynamic analysis, and found that Fe2O3 exhibited the best syngas and steam conversion. The Fe2O3 OC displayed stable redox performance during 10 cycles at 600 °C and possessed a low attrition rate of 0.57% during the redox process in entrained flow reactor [81][156]. When a moving bed reactor was utilized, they found that a syngas conversion of 99.95% with Fe2O3 conversion close to 50% was achieved at 900 °C [82][157]. Based on thermodynamic analysis and packed bed experiments, Aston et al. [83][160] found that the conversion of syngas reached more than 99% during the reduction process of NiFe2O4 and CoFe2O4. Furthermore, the reduced NiFe2O4 (NiFe alloy) and CoFe2O4 (CoFe alloy) was highly reactive towards water-splitting with regeneration of spinel structure, which rendered the NiFe2O4 and CoFe2O4, bearing better redox performance than Fe2O3 (not recovered by water) for SCL process. He et al. [84][161] investigated the effect of preparation methods, including solid-state method, coprecipitation method, hydrothermal method, and sol-gel method, on the SCL performance of NiFe2O4 nanoparticles. It was found that the OC prepared by sol-gel method displayed the best hydrogen yield and highest lattice oxygen recovery degree due to its smaller particle size and porous structure.2.5. Photo-Thermochemical Cycle
In 2016, Zhang and coworkers [23][34] first proposed a strategy of photo-thermochemical cycle (PTC) for CLWS, which explored the photochemical reduction method instead of thermal reduction. This method not only considerably decreased the threshold for abstracting lattice oxygen from OC, slowed down the sintering process, and improved the cyclic stability, but also enabled transformation of solar energy into chemical energy. In a typical photo-thermochemical cycle for water-splitting, the OCs were firstly reduced using ultraviolet and visible light at room temperature with releasing oxygen to generate photo-induced oxygen vacancies, and subsequently recovered by water-splitting via infrared heating to temperature of 500~600 °C with production of hydrogen.3. Summary
Chemical looping water-splitting is promising for sustainable hydrogen production due to the virtue of decoupling a one-step reaction into two or three spatially separated reactions, which greatly simplifies the gas separation process and avoids the harsh conditions for direct water decomposition. To date, different processes have been developed, including a two-step thermochemical water-splitting (TCWS) cycle, methane chemical looping process, chemical looping water gas shift (CL-WGS) cycle, syngas chemical looping (SCL) process, and photo-thermochemical cycle (PTC), with attempts to reduce the energy penalty and CO2 emissions by altering the method to abstract the lattice oxygen from the OCs, wherein the key lies in the manufacture of suitable OCs.
For the two-step TCWS cycle, various OCs, such as iron oxides, zinc oxides, cerium oxides, and perovskite have been widely studied. Among them, cerium oxides have attracted particular attention due to their high structural stability and water-splitting conversion. However, the relatively low reduction degree during the redox cycle, rendering a low hydrogen yield (0.72~7.58 mL/g), greatly hampered its practical applications. Recent work showed that perovskite oxides are promising candidates for two-step TCWS with hydrogen yields of up to 3.13 to 10.71 mL/g, since their redox properties can be facilely modulated by tuning the A/B sites. This gives a clue that constructing composite materials to adjust the redox potential suitable for oxygen desorption and water-splitting should be the key for improving the hydrogen productivity.
Compared to the two-step TCWS cycle, introducing reducing gas, such as methane, carbon monoxide, and syngas, to reduce the oxygen carrier is capable of notably decreasing the reaction temperature to below 1000 °C while enhancing the available oxygen capacity, which significantly decreases the energy consumption, slows down the sintering of OCs, and improve the yield of hydrogen to 13.44~267.63 mL/g. As for methane-driven reduction, valuable syngas with H2/CO ratio of two for Fischer–Tropsch synthesis and methanol production is produced when a suitable OC is selected. Upon OCs with high reducibility applied, the reducing gaseous can be totally combusted with generation of high concentration CO2 (and H2O). All these processes greatly inhibit the side reactions and reduce the burden for gas separation and CO2 caption, rendering improved efficiency and lowered cost for hydrogen production. Among the investigated OCs, iron-based oxides are among the most studied materials due to the virtues of low-cost, environmentally benign features with the high capacity to donate lattice oxygen by varying the valence state of Fe cations.
At present, selection of OCs for CLWS reactions mainly relies on screening method. This is mainly ascribed to the harsh reaction conditions and dynamic structural evolution during redox reactions, which poses a huge challenge for comprehensively understanding the reaction mechanism and designing advanced OCs for CLWS reactions. Future studies should pay more attention to establish a more precise structure–function relationship with the help of in situ characterization, theoretical calculations, and thermodynamic analysis to provide a theoretical basis and development direction for the design of new efficient long-life OCs. Furthermore, according to the pioneering studies, the research focus of OCs is gradually transferred from simple metal oxides to composite oxides (e.g., perovskite) and mixed oxides due to the feasibility of modulating the redox properties by altering the composition of OCs or synergy between different oxides, which bypasses the shortcomings of single metal oxides, and improves the performance of hydrogen production. Therefore, exploring composite oxides to precisely control the metal–oxygen bond strength and mixed oxides to integrate the advantages of different oxides would be an effective strategy for further improving the redox performance of OCs.
