Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Krishnakumar Melethil and Version 2 by Conner Chen.
Supercapacitors are mainly divided into three types: electrical double layer (EDL) capacitors, pseudocapacitors, and hybrid supercapacitors based on their energy storage mechanisms.
- supercapacitors
- water-in-salt
- deep eutectic solvents
1. Supercapacitors with EDLC Behavior
Among other types of SCs, EDLC SCs are the most used for electric vehicles and the storage of electrical energy generated by different energy resources. In EDLC SCs, both water and organic-based electrolytes have been utilized, and a variety of carbonaceous materials have been employed as electrodes. These include activated carbon, amorphous carbon powder, carbon fibers, carbon nanotubes, carbon-based quantum dots, and aerogels [1][20]. For EDLC SCs, the energy is mainly stored via non-faradaic processes (electrostatically) [2][21]. The oppositely charged ions re-orient to generate the Helmholtz double layers at the electrode–electrolyte interface. The potential-dependent surface energy stored at the interface between electrodes generates a double-layer capacitance through electrostatic force (Figure 1a). The nature of CV corresponding to an ideal EDLC behavior that usually exhibits rectangular-shaped curves is shown in Figure 1b. The greater surface area of electrodes and the higher thicknesses of Helmholtz double layers lead to the high capacitance of EDLC SCs. They further possess good durability and cyclic stability over several thousands of charge–discharge cycles [3][22].
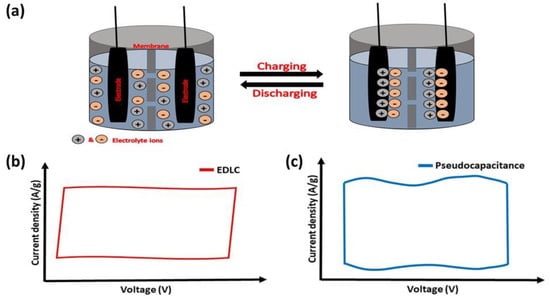
Figure 1. (a) Schematic representation showing the energy storage mechanism of EDLC supercapacitors. Representative CVs of (b) EDLC and (c) pseudocapacitance behaviors.
Graphene’s huge surface area and conductivity make it one of the most often used electrode materials for EDLC SCs [4][23]. The nitrogen doping of graphene materials enhances the electrochemical activity [5][24]. The crumpled nitrogen-doped graphene, for instance, has a maximum capacitance of >300 F g−1 (5 mV s−1) in alkaline electrolytes with a BET surface area of 465 m2 g−1 and pore volume of 3.42 cm3 g−1 [6][25]. A comparative study of nanoporous and nonporous carbon electrodes has demonstrated the important effect of nanoporosity in electrode materials for enhancing electrochemical performances [7][26]. The electrosorption of electrolyte ions over the electrodes is very limited and they interact only at the surface of the nonporous electrode without any transformation in the bulk electrolyte concentration during the charging and discharging. However, the better electrosorption of ions on the porous electrodes improves the power density and lifetime of EDLC SCs. To be more environmentally friendly, carbon electrodes can be prepared from biowaste as the precursor material. For example, the carbon material is synthesized by the pyrolysis of waste banana fibers and subsequently treated with KOH and ZnCl2 to create porosity [8][27]. This process increases the activated carbon electrode surface area by up to 30 times more than that of the untreated material. The resulting carbon electrode shows a specific capacitance of ~70 F g−1 (500 mA g−1) and better Coulombic efficiency (>85%) with a high current density of over 500 cycles. Moreover, mesoporous carbon materials with cylinder and gyroid nanostructures are synthesized by using amphiphilic polyethylene oxide-block-caprolactone (PEO-PCL) as a template [9][28]. The prepared cylinder (135 F g−1) and gyroid-shaped (155 F g−1) carbon materials show better capacitance values in 6 M KOH electrolyte. Furthermore, these two materials show 96 and 105.6 F g−1 in 1M tetraethylammonium tetrafluoroborate, respectively [9][28].
2. Pseudocapacitance-Based Supercapacitors
The basic mechanism of energy storage in pseudocapacitors involves redox reactions on the electrode surface [10][29]. In general, pseudocapacitors display 10–100 times higher specific capacitance than EDLC devices because of faradaic charge transfer taking place in the active materials. These types of electrode materials show near-rectangular CVs with broader peaks (for example, as shown in Figure 1c) due to the surface-confined very fast electron transfer reactions. Many transition-metal oxides and conducting polymers exhibit pseudocapacitive properties. The ability of transition-metal oxides to exhibit multiple valence states enables them to exhibit pseudocapacitive behavior [11][12][30,31]. In addition, manganese dioxide is one of the best-performing pseudocapacitor active electrodes among various metal oxides and the effect of different phases and morphology of MnO2-based electrodes have been extensively reported. For example, MnO2 with different phases (α-, β-, and γ-phase) have been prepared through coupled microwave–hydrothermal reaction in the MnCl2−KMnO4 aqueous solution system. The plate-shaped γ-phase with a trace of β-MnO2 shows better performance in capacitance studies [13][32]. Similarly, ruthenium dioxide (RuO2) is a highly conductive metal oxide that has garnered attention for its exceptional electrochemical reversibility, high capacitance, and long lifespan [14][33]. However, less surface area is considered one of the demerits of ruthenium dioxide. It has been overcome by preparing the different nanostructures of RuO2. For instance, the hydrothermally prepared RuO2-based porous structures provide a greater surface area of 159.4 m2 g−1. The RuO2 electrode delivers a capacitance of 400 F g−1 (0.2 A g−1) and maintains a capacitance retention of 84.7% after 6000 GCD cycles (10 A g−1) [15][34]. Moreover, the other metal oxides of copper, cobalt, zinc, titanium, iron, and tungsten also exhibit great performances as active electrode materials in pseudocapacitors [16][17][18][19][20][21][35,36,37,38,39,40]. Metal oxides that include several different metal elements have recently drawn a lot of interest as electrode materials for pseudocapacitors. For example, the NiO and MnO2-modified V2O5 nanoribbon is an example of a ternary metal oxide electrode material. The nanoribbon improves energy (138 Wh kg−1) and power (450 W kg−1) densities with an extended lifespan (retaining 83.6% after 10,000 cycles), and high capacitance (788 F g−1 at 5 mV s−1). The above characteristics result from the reactive surfaces with interpenetrating channels of electrode material, which promote effective electron and ion conduction [22][41].
In the field of electrochemistry, polyaniline has been the focus of substantial research and it is regarded as a useful polymer for supercapacitor applications due to its outstanding electrochemical response, which includes multi-redox behavior, flexibility, and low cost [23][24][42,43]. The electrochemical performances of PANI-based electrodes are increased by engineering their morphology, doping with heteroatoms, or forming composites with conductive carbon [25][26][27][28][29][44,45,46,47,48]. Likewise, polypyrrole (PPy) and its composites show interesting redox properties as well as good electrical conductivity [30][31][32][33][49,50,51,52]. However, the practical applications of PANI and PPy polymers are limited by their poor cyclic stability due to the repeated volumetric swelling and shrinkage during GCD experiments. The instability of the conducting polymer has recently been overcome by coating the conducting polymer with thin carbonaceous shells [34][53].
3. Hybrid Supercapacitors
Hybrid supercapacitors (HSC) provide better energy density, power density, and life span than the individual components [35][36][54,55]. HSCs are the combinations of non-faradaic EDLC and faradaic pseudocapacitance [37][38][56,57]. In general, a carbon electrode and metal oxide are used as an EDLC and pseudocapacitive electrode in the HSC configuration, respectively. Figure 2 shows the device structure of an HSC made of 3D graphene hydrogel (GH) and the MnO2 nanoflakes over a nickel foam as the anode and cathode, respectively. A metal oxide with high intrinsic capacitance provides good energy density, whereas the carbon electrode supplies high power density. As a result, this HSC exhibits a 23.2 Wh kg−1 energy density and a 1.0 kW kg−1 power density [39][58].
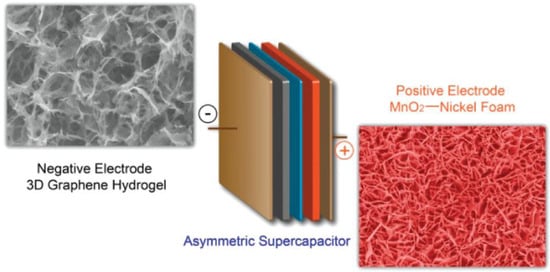
Figure 2.
Graphical representation of an HSC device with graphene hydrogel and MnO
A hybrid device comprising graphene oxynitride (GON) electrodes exhibits a capacitance of 783.5 F g at a current density of 1 A g−1. The GON electrodes are produced over a low-temperature hydrothermal route with an ammonia solution. This preparation method results in the formation of quaternary, pyrrolic, pyridinic, and pyridinic-N-oxides on graphene oxide (GO), which not only improves the capacitance owing to their positive charges but also enhances the electron transfer within the GO. A GON-based HSC exhibits a good cyclability of 5000 cycles with an 87% efficiency in PVA/KOH gel electrolytes and produces a 40 Wh kg−1 energy density at a 900 W kg−1 power density [40][59].
The electrode in the HSC can also exhibit battery-like behavior where a pair of redox peaks is usually observed in its CV curve. The redox reaction in this instance takes place in the electrode and is kinetically regulated by diffusion. For example, Co3O4-decorated P, N-co-doped porous carbon material (PNC) is one of the representative examples of battery-like electrodes. When the electrode is combined with activated carbon (anode), the resulting HSC with a 6 M KOH electrolyte demonstrates better energy density (47.18 Wh g−1), power density (375 W kg−1), and possesses a capacitance retention of 92% (5000 GCD cycles). The electrochemical performance is improved because of the higher surface area resulting from the porous structures of PNC and the small particle size of cobalt oxide. Furthermore, the strong anchoring effect of PNC on Co3O4 nanoparticles, along with the confinement effect of the nanocavities, helps to maintain the stability of the supercapacitor [41][60]. Similarly, the flower-like mesoporous NiCo2O4 structures synthesized using the solvothermal method can serve as a battery-type behavior that exhibits a 122.5 C g−1 specific capacity at 1 A g−1 in a 6M KOH electrolyte. This electrode maintains decent cycling stability, with a loss of just 21% in specific capacity over 6000 cycles at 2 A/g, and demonstrates an improved electrochemical performance with an increase in the concentration of aqueous electrolyte [42][61].
