Allergic inflammation is a type 2 immune disorder traditionally treated with epinephrine and glucorticosteroids in the short term. In extreme cases, current treatment options include the use of monoclonal antibodies targeting the pathological pathways of inflammation. However, this is impeded by the cost of production, host immunogenicity, safety, and the efficacy of these antibodies, and by their availability in developing countries. The use of purified monoclonal antibodies for treating severe allergic responses and the associated limitations are described.
- allergy
- inflammation
- gene
- antibodies
1. Introduction
2. Mechanism of an Allergic Reaction
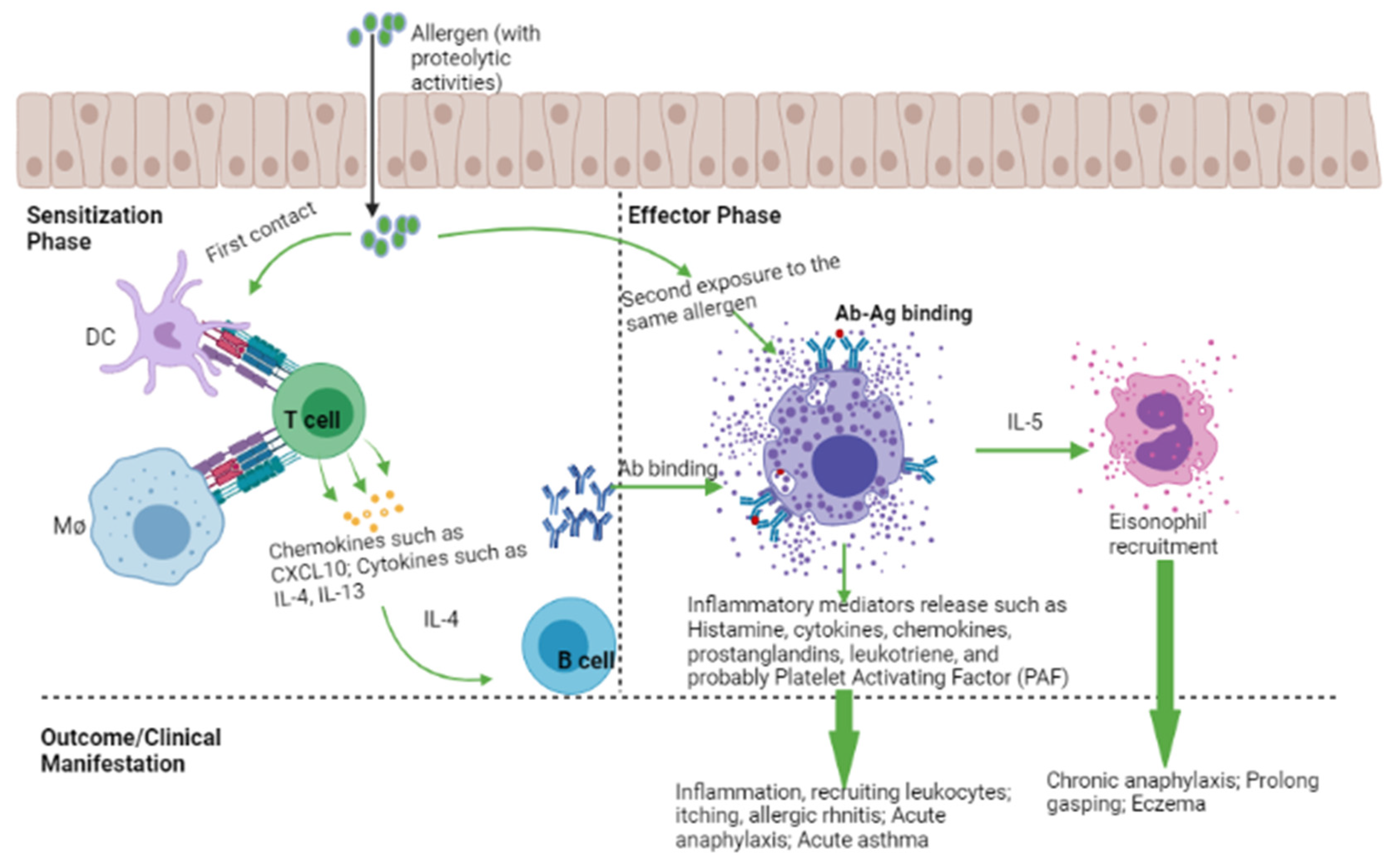
3. Histamine, Histamine Receptors, and Clinical Outcome
| Receptors | Effects | Clinical Manifestation | References |
|---|---|---|---|
| H1 | Stimulates nociceptive nerve fiber; bronchoconstriction; increase mucus secretion | Itching; urticaria; allergic rhinoconjunctivitis; allergic asthma | [15][17] |
| H2 | Gastric acid secretion; arrhythmia; increased intracellular cAMP |
Peptic ulcer anaphylaxis; negative feedback on mast cell activation |
[15][20] |
| H3 | Inflammation on the neurons | Neuro-inflammatory diseases such as epilepsy; CNS disorder |
[15][21] |
| H4 | Induce eosinophil shape change; activates eosinophils | Lung asthma | [19] |
4. Possible tTherapeutic tTargets that aAlleviate aAllergic dDiseases
The search for an effective therapeutic target against endotype-specific markers (such as free serum IgE, and cytokines) in allergic asthma has long been an active area of allergology research, and most developed biologics are still in clinical trials. Inhaled corticosteroids (ICS) are the first line-medication for both asthmatic children [28] and adults [29]. ICS helps to modulate blood eosinophil levels [29] and improve lung function. The drawback of this treatment is its inherent heterogeneity and patients’ genetic variation [30], toxicity [31][32], such as adrenal complications, muscle weakness, osteoporosis [33], and impaired immunity against pneumonia and tuberculosis [34]. In April 2019, the Global Initiative for Asthma (GINA), no longer recommended the use of asthma-only short-term bronchodilators in its guidelines [35]. This is because the administration of only beta-2 agonist bronchodilators increases the risk for asthma and its comorbidities such as hypertension [36].
4.1. Targeting IgE in Allergic Inflammation
Omalizumab is now used to treat moderate-to-severe asthma patients where bronchodilators and ICS have failed [37][38][39]. Omalizumab is a recombinant humanized mAb that suppresses both the early and the late asthmatic responses by preventing the interaction of serum IgE with FcεR1 on mast cells, blood basophils, and eosinophils [40][41]. Omalizumab has also been reported to effectively reduce respiratory symptoms associated with allergic asthma in a randomized control trial [42]. Likewise, Esquivel et al. [43] reported that omalizumab reduces serum IgE levels and promotes viral load clearance in asthmatic children infected with rhinovirus. In a separate study, omalizumab was found to reduce platelets and leukocytes count, and C-reactive protein (CRP) levels in chronic urticaria patients [44]. Furthermore, omalizumab was shown to substantially reduce nasal and bronchial mucosal inflammation in patients with rhinitis experiencing severe allergic asthma [45]. Omalizumab is also promising in older patients with asthma-associated COPD [46]. Patients receiving omalizumab show improved asthma control test (ACT) scores accompanied by a reduced number of exacerbations [46].4.2. Targeting Th2-Associated Cytokines in Allergic Disease
IL-4 is a crucial cytokine involved in the differentiation of naïve CD4+ T cells into Th-2 effector cells, and it is an essential signature of type II inflammatory response [5][47]. Murine model studies have revealed that IL-4 but not IL-5 is central to both inducing Th-2 cell activation/response and airway eosinophilic recruitment/inflammation [48][49]. Blockade of IL-4 is a possible target for alleviating most allergic diseases such as asthma, rhinitis, and eczema. Studies have shown that both altrakincept and pascolizumab reduce the recruitment of eosinophils at the site of allergic inflammation by masking the patient’s serum IL-4 (Figure 2), but with low clinical efficacy [31][50][51]. Both altrakincept and pascolizumab could not make it through phase III clinical trials and, as such, were suspended from being launched into the market [52]. Similar to IL-4, IL-13 is also a central mediator of allergic inflammation. IL-13 can induce most of the key characteristic features of experimental asthma and allergy, including allergen-induced airway hypersensitivity, goblet cell hyperplasia with mucus hyper-production, and eosinophilia [53][54][55]. Lebrikizumab is a humanized mAb that blocks IL-13 functionality (Figure 2) [31][56].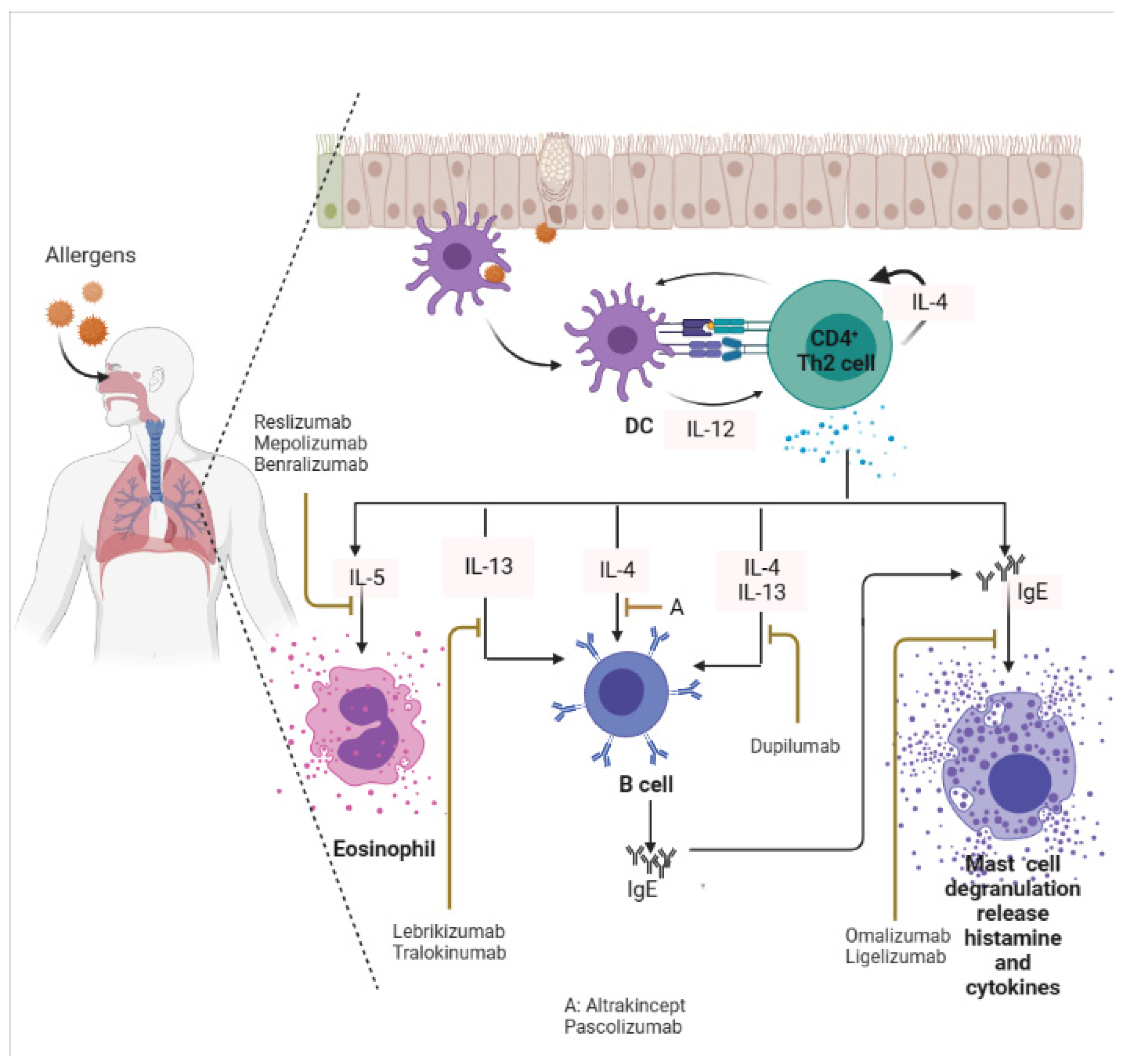 Figure 2. Targeting IgE, and Th-2 (IL-4, IL-5, IL-13) in an allergic inflammatory response. Monoclonal antibodies that target these biomarkers can alleviate symptoms associated with allergies. DC: Dendritic cells. This image was created in bio render.
Figure 2. Targeting IgE, and Th-2 (IL-4, IL-5, IL-13) in an allergic inflammatory response. Monoclonal antibodies that target these biomarkers can alleviate symptoms associated with allergies. DC: Dendritic cells. This image was created in bio render.
4.3. Monoclonal Antibodies Targeting Dual Inflammatory Mediators
Emerging reports on the development of mAb targeting multiple Th-2 cytokine responses during allergy episodes have achieved a milestone. For example, Kasaian et al. [57] developed a murine IL-4/IL-13 antagonist that efficiently neutralizes IL-4 and IL-13, as well as reduces serum IgE, lung eosinophilia, lung Mu5ac expression, and airway resistance in OVA-challenged mice. Dupilumab is the only approved mAb with the capability to inhibit the IL-4/IL-13 pathway in patients with atopic dermatitis [58], and allergic asthma [59], via IL-4Rα blockade [60]. Dupilumab neutralizes IL-4 and IL-13 cytokines (Figure 2). Dupilumab received this approval because it improves the quality of patient’s life where other treatments had failed [58]. It has also been reported that dupilumab modulates eosinophils infiltration, B-cells activation, Th2cell-driven dendritic cell activation and blocks the expression of pro-inflammatory cytokines (Il4, Il13, Il5, Il1α) and chemokines in mouse asthma model [61]. Further, Jonstam et al. [62] reported that dupilumab reduces type 2 inflammatory biomarkers such as serum IgE, and eosinophil chemokine release in patients suffering from multiple allergic chronic rhino-sinusitis with nasal polyposis.4.4. Setbacks and Limitations
Despite the successes in the current mAb therapy for mild-to-severe allergy, there is a range of side effects associated with mAb intake. These include serum sickness, headaches, mild gastrointestinal symptoms, itching, cardiac toxicity, and anaphylaxis which could be life-threatening [63]. For example, a side effect of omalizumab is the induction of immunogenicity and anaphylaxis [64][65]. Other limitations include the inconsistencies in mAb, the cost of mAb production, including its purification, efficacy, and safety [66][67], as well as the skewed results from inconclusive clinical trials. Taken together, cost-effectiveness, inherent factors, and host genetic variation play a crucial role in utilizing mAb in mild-to-severe allergy treatment.5. Antibody Gene Therapy: The Future for Antibody Therapy
The transfer of genes into host cells began in the late quarter of the 20th century using suitable vectors such as the Adeno-associated virus (AAV) vector, which were thought not to be associated with any illness in the human population [68][69]. Ever since then, it became possible to transfer mAb and/or any therapeutic proteins of interest using an AAV vector, and the use of this approach in several studies has been well-described [70][71]. The use of AVV coding antibody to reduce allergic events have been extensively studied in animal models [72][73][74]. For example, AAV vector coding for high-affinity anti-IgE reduces IgE-mediated peanut histamine release and anaphylaxis score in NOD scid gamma mice [73]. However, this approach became a major concern when Nault et al. [75] reported clonal integration of AAV genomes in tumor-driver genes of hepatocellular carcinomas, suggesting that the use of AAV may induce mutagenesis. Other setbacks include the possibility of the host developing an immune response against AAV capsid in patients who had been predisposed to the wild-type AAV [68]. The involvement of Toll-like receptor (TLR)9 and MyD88-mediated pathways in CD8+ T cells mediated responses to AAV-mediated gene transfer in mice had already been reported [76]. Emerging clinical trials also show similar findings of the host inducing an immune response to AAV [77][78]. These discoveries led to the birth of an alternative route of producing and delivering antibodies with fewer challenges. Studies on the delivery of mRNA-mediated antibody gene into host cells for passive immunity against pathogenic infection, vaccination against tumor growth, and allergy management are still evolving. In contrast to the manufacturing of purified monoclonal antibodies for severe allergy treatment, mRNA-directed antibody therapy can be cost-effective and safe and might only require a single local or systemic targeted shot to exert its therapeutic function [79][80]. Currently, there are only a few preclinical studies on mRNA-encoding antibodies [80]. The use of mRNA-mediated antibodies against viral infection has been extensively studied [79][81]. Thran et al. [79] reported that a single shot of mRNA-LNP encoding anti-rabies antibody provides complete protection against rabies infection even when pre-exposed and/or after several post-exposed challenges with rabies virus. The authors [79] further compared the efficacy of mRNA-LNP encoding rituximab to that of recombinant rituximab administration on tumor growth and found that the anti-tumor effect of mRNA-LNP encoding rituximab was higher than recombinant rituximab. This suggests a promising therapeutic option for the future. Further, Pardi et al. [81] also reported that a single injection of a modified mRNA encoding the light and heavy chain of an anti-HIV1 antibody, VRC01, in mice confers full protection against SF162 and JR-CSF HIV-1 isolates challenge. This suggests that the mRNA-LNP encoding VRCO1 used in their study successfully integrated into host cells and directs the synthesis of broadly neutralizing anti-HIV antibody, VRCO1, capable of conferring host-passive immunity against HIV-1 infection [81]. To date, there are limited/or no studies on the use of mRNA-encoding antibodies for protection against allergy. The current literature on mRNA encoding antibodies on other disease models has been described elsewhere [82][83]. The application of mRNA as a vaccine has also been successful in conferring protection against the dreadful SARS-CoV-2 virus. Despite this, a few challenges may affect antibody gene therapy for allergy. These include: (1) the potential dangers associated with implanting genes into human hosts to express antibodies against host-inherent allergy biomarkers; (2) this implanted gene may induce endogenous pathogenic viruses’ reactivation and mutagenesis; (3) problems related to large-scale good manufacturing practice production; (4) the half-life of the antibodies encoded by mRNA; and (5) the safety and efficacy of antibody gene therapy for allergies in humans have not translated to its use clinically. Gene therapy in allergy is an emerging and exciting field, but with limited data. Hopefully, future studies on mRNA encoding either monospecific or bispecific antibodies against allergy biomarkers will continue to evolve and improve in the coming years.6. Conclusions
Monoclonal antibodies that target serum IgE and Th-2 cytokines have shown potential to reduce the severity of allergic asthma, atopic dermatitis, and rhinitis. However, the therapeutic use of purified mAb faces several challenges, such as the heterogeneity of allergy phenotypes, the efficacy and safety of mAb in clinical trials, the high cost of mAb production, and the limited availability of mAb. Gene therapy using AAV vector was initially promising, but later revealed adverse effects, such as host immune response against AAV. This led to the exploration of mRNA-mediated antibody gene therapy as a novel approach for antibody delivery. The mRNA-mediated antibody has several advantages over conventional mAb, such as requiring only a single injection and periodic boosters. Therefore, future research should focus on developing and optimizing mRNA-mediated antibody gene therapy for a wide range of atopic diseases/allergiesReferences
- Kubo, M. Innate and Adaptive Type 2 Immunity in Lung Allergic Inflammation. Immunol. Rev. 2017, 278, 162–172.
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The Development of Allergic Inflammation. Nature 2008, 454, 445–454.
- Yoshimoto, T.; Tsutsui, H.; Tominaga, K.; Hoshino, K.; Okamura, H.; Akira, S.; Paul, W.E.; Nakanishi, K. IL-18, Although Antiallergic When Administered with IL-12, Stimulates IL-4 and Histamine Release by Basophils. Proc. Natl. Acad. Sci. USA 1999, 96, 13962–13966.
- Barnes, P.J. Pathophysiology of Allergic Inflammation. Immunol. Rev. 2011, 242, 31–50.
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888.
- Dvorin, E.L.; Ebell, M.H. Short-Term Systemic Corticosteroids: Appropriate Use in Primary Care. Am. Fam. Physician 2020, 101, 89–94.
- Venekamp, R.P.; Thompson, M.J.; Hayward, G.; Heneghan, C.J.; Mar, C.B.D.; Perera, R.; Glasziou, P.P.; Rovers, M.M. Systemic Corticosteroids for Acute Sinusitis. Cochrane Database Syst. Rev. 2014.
- Aasbjerg, K.; Torp-Pedersen, C.; Vaag, A.; Backer, V. Treating Allergic Rhinitis with Depot-Steroid Injections Increase Risk of Osteoporosis and Diabetes. Respir. Med. 2013, 107, 1852–1858.
- Hox, V.; Lourijsen, E.; Jordens, A.; Aasbjerg, K.; Agache, I.; Alobid, I.; Bachert, C.; Boussery, K.; Campo, P.; Fokkens, W.; et al. Benefits and Harm of Systemic Steroids for Short- and Long-Term Use in Rhinitis and Rhinosinusitis: An EAACI Position Paper. Clin. Transl. Allergy 2020, 10, 1.
- Choo, K.J.L.; Simons, E.; Sheikh, A. Glucocorticoids for the Treatment of Anaphylaxis: Cochrane Systematic Review. Allergy 2010, 65, 1205–1211.
- Ring, J.; Gutermuth, J. 100 Years of Hyposensitization: History of Allergen-Specific Immunotherapy (ASIT). Allergy 2011, 66, 713–724.
- Durham, S.R.; Nelson, H. Allergen Immunotherapy: A Centenary Celebration. World Allergy Organ. J. 2011, 4, 104–106.
- He, S.; Zhang, H.; Zeng, X.; Chen, D.; Yang, P. Mast Cells and Basophils Are Essential for Allergies: Mechanisms of Allergic Inflammation and a Proposed Procedure for Diagnosis. Acta Pharmacol. Sin. 2013, 34, 1270–1283.
- Kamijo, S.; Takeda, H.; Tokura, T.; Suzuki, M.; Inui, K.; Hara, M.; Matsuda, H.; Matsuda, A.; Oboki, K.; Ohno, T. IL-33–Mediated Innate Response and Adaptive Immune Cells Contribute to Maximum Responses of Protease Allergen–Induced Allergic Airway Inflammation. J. Immunol. 2013, 190, 4489–4499.
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873.
- Albrecht, M.; Dittrich, A.M. Expression and Function of Histamine and Its Receptors in Atopic Dermatitis. Mol. Cell. Pediatr. 2015, 2, 16.
- Bakker, R.A.; Schoonus, S.B.; Smit, M.J.; Timmerman, H.; Leurs, R. Histamine H1-Receptor Activation of Nuclear Factor-ΚB: Roles for Gβγ-and Gαq/11-Subunits in Constitutive and Agonist-Mediated Signaling. Mol. Pharmacol. 2001, 60, 1133–1142.
- Wernersson, S.; Pejler, G. Mast Cell Secretory Granules: Armed for Battle. Nat. Rev. Immunol. 2014, 14, 478–494.
- Buckland, K.F.; Williams, T.J.; Conroy, D.M. Histamine Induces Cytoskeletal Changes in Human Eosinophils via the H4 Receptor. Br. J. Pharmacol. 2003, 140, 1117–1127.
- Seifert, R.; Strasser, A.; Schneider, E.H.; Neumann, D.; Dove, S.; Buschauer, A. Molecular and Cellular Analysis of Human Histamine Receptor Subtypes. Trends Pharmacol. Sci. 2013, 34, 33–58.
- Singh, M.; Jadhav, H.R. Histamine H3 Receptor Function and Ligands: Recent Developments. Mini Rev. Med. Chem. 2013, 13, 47–57.
- Lippert, U.; Artuc, M.; Grützkau, A.; Babina, M.; Guhl, S.; Haase, I.; Blaschke, V.; Zachmann, K.; Knosalla, M.; Middel, P.; et al. Human Skin Mast Cells Express H2 and H4, but Not H3 Receptors. J. Investig. Dermatol. 2004, 123, 116–123.
- Xu, J.; Zhang, X.; Qian, Q.; Wang, Y.; Dong, H.; Li, N.; Qian, Y.; Jin, W. Histamine Upregulates the Expression of Histamine Receptors and Increases the Neuroprotective Effect of Astrocytes. J. Neuroinflammation 2018, 15, 41.
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13.
- Dong, H.; Zhang, X.; Wang, Y.; Zhou, X.; Qian, Y.; Zhang, S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol. Neurobiol. 2017, 54, 997–1007.
- Dinh, Q.T.; Cryer, A.; Dinh, S.; Peiser, C.; Wu, S.; Springer, J.; Hamelmann, E.; Klapp, B.F.; Heppt, W.; Fischer, A. Transcriptional Up-Regulation of Histamine Receptor-1 in Epithelial, Mucus and Inflammatory Cells in Perennial Allergic Rhinitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2005, 35, 1443–1448.
- Merves, J.; Chandramouleeswaran, P.M.; Benitez, A.J.; Muir, A.B.; Lee, A.J.; Lim, D.M.; Dods, K.; Mehta, I.; Ruchelli, E.D.; Nakagawa, H.; et al. Altered Esophageal Histamine Receptor Expression in Eosinophilic Esophagitis (EoE): Implications on Disease Pathogenesis. PLoS ONE 2015, 10, e0114831.
- Wu, Y.-F.; Su, M.-W.; Chiang, B.-L.; Yang, Y.-H.; Tsai, C.-H.; Lee, Y.L. A Simple Prediction Tool for Inhaled Corticosteroid Response in Asthmatic Children. BMC Pulm. Med. 2017, 17, 1–6.
- Rhyou, H.-I.; Nam, Y.-H. Predictive Factors of Response to Inhaled Corticosteroids in Newly Diagnosed Asthma: A Real-World Observational Study. Ann. Allergy. Asthma. Immunol. 2020, 125, 177–181.
- Keskin, O.; Farzan, N.; Birben, E.; Akel, H.; Karaaslan, C.; Maitland-van der Zee, A.H.; Wechsler, M.E.; Vijverberg, S.J.; Kalayci, O. Genetic Associations of the Response to Inhaled Corticosteroids in Asthma: A Systematic Review. Clin. Transl. Allergy 2019, 9, 2.
- Kau, A.L.; Korenblat, P.E. Anti-Interleukin 4 and 13 for Asthma Treatment in the Era of Endotypes. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 570.
- Nabe, T. Interleukin (IL)-33: New Therapeutic Target for Atopic Diseases. J. Pharmacol. Sci. 2014, 126, 85–91.
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of Long-Term Oral Corticosteroid Therapy and Its Side-Effects in Severe Asthma in Adults: A Focused Review of the Impact Data in the Literature. Eur. Respir. J. 2018, 52, 1800703.
- Kaur, S.; Singh, V. Asthma and Medicines–Long-Term Side-Effects, Monitoring and Dose Titration. Indian, J. Pediatr. 2018, 85, 748–756.
- Reddel, H.K.; FitzGerald, J.M.; Bateman, E.D.; Bacharier, L.B.; Becker, A.; Brusselle, G.; Buhl, R.; Cruz, A.A.; Fleming, L.; Inoue, H. GINA 2019: A Fundamental Change in Asthma Management: Treatment of Asthma with Short-Acting Bronchodilators Alone Is No Longer Recommended for Adults and Adolescents. Eur. Respir. J. 2019, 53, 1901046.
- Eff, A.R.Y. Incidence of Hypertension in Asthma Patients Who Treated with Beta-2 Agonists Bronchodilators. Int J Pharm Pharm Sci 2017, 9, 181–184.
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, Anti-IgE Recombinant Humanized Monoclonal Antibody, for the Treatment of Severe Allergic Asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190.
- Deleanu, D.; Nedelea, I. Biological Therapies for Atopic Dermatitis: An Update. Exp. Ther. Med. 2019, 17, 1061–1067.
- Holgate, S.; Bousquet, J.; Wenzel, S.; Fox, H.; Liu, J.; Castellsague, J. Efficacy of Omalizumab, an Anti-Immunoglobulin E Antibody, in Patients with Allergic Asthma at High Risk of Serious Asthma-Related Morbidity and Mortality. Curr. Med. Res. Opin. 2001, 17, 233–240.
- Fahy, J.V.; Fleming, H.E.; Wong, H.H.; Liu, J.T.; Su, J.Q.; Reimann, J.; Fick, R.B., Jr.; Boushey, H.A. The Effect of an Anti-IgE Monoclonal Antibody on the Early- and Late-Phase Responses to Allergen Inhalation in Asthmatic Subjects. Am. J. Respir. Crit. Care Med. 1997, 155.
- Trischler, J.; Lieb, A.; Arnold, M.; Schulze, J.; Rosewich, M.; Schubert, R.; Bottoli, I.; Zielen, S. Omalizumab Effectively Protects against Early and Late Allergic Responses in Asthma after 4 Weeks. Allergy 2017, 72, 1912–1915.
- Pelaia, G.; Canonica, G.W.; Matucci, A.; Paolini, R.; Triggiani, M.; Paggiaro, P. Targeted Therapy in Severe Asthma Today: Focus on Immunoglobulin, E. Drug Des. Devel. Ther. 2017, 11, 1979.
- Esquivel, A.; Busse, W.W.; Calatroni, A.; Togias, A.G.; Grindle, K.G.; Bochkov, Y.A.; Gruchalla, R.S.; Kattan, M.; Kercsmar, C.M.; Khurana Hershey, G. Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 985–992.
- Acer, E.; Kaya Erdogan, H.; Yüksel Çanakçı, N.; Saracoglu, Z.N. The Effect of Omalizumab on Hematological and Inflammatory Parameters in Patients with Chronic Spontaneous Urticaria. Cutan. Ocul. Toxicol. 2019, 38, 5–8.
- Masieri, S.; Cavaliere, C.; Begvarfaj, E.; Rosati, D.; Minni, A. Effects of Omalizumab Therapy on Allergic Rhinitis: A Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5249–5255.
- Ricciardi, L.; Papia, F.; Liotta, M.; Cicero, F.; Isola, S.; Tartarisco, G.; Furci, F.; Gangemi, S. Omalizumab in Middle-Aged or Older Patients with Severe Allergic Asthma-COPD Overlap. Adv. Dermatol. Allergol. Dermatol. Alergol. 2022, 39, 88–93.
- Saggini, A.; Maccauro, G.; Tripodi, D.; De Lutiis, M.A.; Conti, F.; Felaco, P.; Fulcheri, M.; Galzio, R.; Caraffa, A.; Antinolfi, P. Allergic Inflammation: Role of Cytokines with Special Emphasis on IL-4; SAGE Publications Sage UK: London, UK, 2011.
- Corry, D.B.; Folkesson, H.G.; Warnock, M.L.; Erle, D.J.; Matthay, M.A.; Wiener-Kronish, J.P.; Locksley, R.M. Interleukin 4, but Not Interleukin 5 or Eosinophils, Is Required in a Murine Model of Acute Airway Hyperreactivity. J. Exp. Med. 1996, 183, 109–117.
- Coyle, A.J.; Le Gros, G.; Bertrand, C.; Tsuyuki, S.; Heusser, C.H.; Kopf, M.; Anderson, G.P. Interleukin-4 Is Required for the Induction of Lung Th2 Mucosal Immunity. Am. J. Respir. Cell Mol. Biol. 1995, 13, 54–59.
- Canonica, G.W.; Senna, G.; Mitchell, P.D.; O’Byrne, P.M.; Passalacqua, G.; Varricchi, G. Therapeutic Interventions in Severe Asthma. World Allergy Organ. J. 2016, 9, 40.
- Heck, S.; Nguyen, J.; Le, D.-D.; Bals, R.; Dinh, Q.T. Pharmacological Therapy of Bronchial Asthma: The Role of Biologicals. Int. Arch. Allergy Immunol. 2015, 168, 241–252.
- Akdis, C.A. Therapies for Allergic Inflammation: Refining Strategies to Induce Tolerance. Nat. Med. 2012, 18, 736–749.
- Vladich, F.D.; Brazille, S.M.; Stern, D.; Peck, M.L.; Ghittoni, R.; Vercelli, D. IL-13 R130Q, a Common Variant Associated with Allergy and Asthma, Enhances Effector Mechanisms Essential for Human Allergic Inflammation. J. Clin. Investig. 2005, 115, 747–754.
- Walter, D.M.; McIntire, J.J.; Berry, G.; McKenzie, A.N.; Donaldson, D.D.; DeKruyff, R.H.; Umetsu, D.T. Critical Role for IL-13 in the Development of Allergen-Induced Airway Hyperreactivity. J. Immunol. 2001, 167, 4668–4675.
- Wang, I.-M.; Lin, H.; Goldman, S.J.; Kobayashi, M. STAT-1 Is Activated by IL-4 and IL-13 in Multiple Cell Types. Mol. Immunol. 2004, 41, 873–884.
- Scheerens, H.; Arron, J.R.; Zheng, Y.; Putnam, W.S.; Erickson, R.W.; Choy, D.F.; Harris, J.M.; Lee, J.; Jarjour, N.N.; Matthews, J.G. The Effects of Lebrikizumab in Patients with Mild Asthma Following Whole Lung Allergen Challenge. Clin. Exp. Allergy 2014, 44, 38–46.
- Kasaian, M.T.; Marquette, K.; Fish, S.; DeClercq, C.; Agostinelli, R.; Cook, T.A.; Brennan, A.; Lee, J.; Fitz, L.; Brooks, J. An IL-4/IL-13 Dual Antagonist Reduces Lung Inflammation, Airway Hyperresponsiveness, and IgE Production in Mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 37–46.
- Lawrence, M.G.; Steinke, J.W.; Borish, L. Cytokine-Targeting Biologics for Allergic Diseases. Ann. Allergy. Asthma. Immunol. 2018, 120, 376–381.
- Corren, J.; Castro, M.; Chanez, P.; Fabbri, L.; Joish, V.N.; Amin, N.; Graham, N.M.H.; Mastey, V.; Abbé, A.; Taniou, C.; et al. Dupilumab Improves Symptoms, Quality of Life, and Productivity in Uncontrolled Persistent Asthma. Ann. Allergy. Asthma. Immunol. 2019, 122, 41–49.e2.
- Olin, J.T.; Wechsler, M.E. Asthma: Pathogenesis and Novel Drugs for Treatment. BMJ 2014, 349, g5517.
- Le Floch, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual Blockade of IL-4 and IL-13 with Dupilumab, an IL-4Rα Antibody, Is Required to Broadly Inhibit Type 2 Inflammation. Allergy 2020, 75, 1188–1204.
- Jonstam, K.; Swanson, B.N.; Mannent, L.P.; Cardell, L.-O.; Tian, N.; Wang, Y.; Zhang, D.; Fan, C.; Holtappels, G.; Hamilton, J.D.; et al. Dupilumab Reduces Local Type 2 Pro-Inflammatory Biomarkers in Chronic Rhinosinusitis with Nasal Polyposis. Allergy 2019, 74, 743–752.
- Baldo, B.A. Adverse Events to Monoclonal Antibodies Used for Cancer Therapy. Oncoimmunology 2013, 2, e26333.
- Cox, L.S. How Safe Are the Biologicals in Treating Asthma and Rhinitis? Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2009, 5, 4.
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338.
- Wu, A.C.; Fuhlbrigge, A.L.; Robayo, M.A.; Shaker, M. Cost-Effectiveness of Biologics for Allergic Diseases. J. Allergy Clin. Immunol. Pract. 2021, 9, 1107–1117.
- Samaranayake, H.; Wirth, T.; Schenkwein, D.; Räty, J.K.; Ylä-Herttuala, S. Challenges in Monoclonal Antibody-Based Therapies. Ann. Med. 2009, 41, 322–331.
- Mingozzi, F.; High, K.A. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood 2013, 122, 23–36.
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378.
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. Biodrugs 2017, 31, 317–334.
- Xu, X.; Chen, W.; Zhu, W.; Chen, J.; Ma, B.; Ding, J.; Wang, Z.; Li, Y.; Wang, Y.; Zhang, X. Adeno-associated Virus (AAV)-Based Gene Therapy for Glioblastoma. Cancer Cell Int. 2021, 21, 76.
- Camilleri, A.E.; Nag, S.; Russo, A.R.; Stiles, K.M.; Crystal, R.G.; Pagovich, O.E. Gene Therapy for a Murine Model of Eosinophilic Esophagitis. Allergy 2021, 76, 2740–2752.
- Pagovich, O.E.; Wang, B.; Chiuchiolo, M.J.; Kaminsky, S.M.; Sondhi, D.; Jose, C.L.; Price, C.C.; Brooks, S.F.; Mezey, J.G.; Crystal, R.G. Anti-HIgE Gene Therapy of Peanut-Induced Anaphylaxis in a Humanized Murine Model of Peanut Allergy. J. Allergy Clin. Immunol. 2016, 138, 1652–1662.e7.
- Zavorotinskaya, T.; Tomkinson, A.; Murphy, J.E. Treatment of Experimental Asthma by Long-Term Gene Therapy Directed against IL-4 and IL-13. Mol. Ther. J. Am. Soc. Gene Ther. 2003, 7, 155–162.
- Nault, J.-C.; Datta, S.; Imbeaud, S.; Franconi, A.; Mallet, M.; Couchy, G.; Letouzé, E.; Pilati, C.; Verret, B.; Blanc, J.-F.; et al. Recurrent AAV2-Related Insertional Mutagenesis in Human Hepatocellular Carcinomas. Nat. Genet. 2015, 47, 1187–1193.
- Rogers, G.L.; Suzuki, M.; Zolotukhin, I.; Markusic, D.M.; Morel, L.M.; Lee, B.; Ertl, H.C.J.; Herzog, R.W. Unique Roles of TLR9- and MyD88-Dependent and -Independent Pathways in Adaptive Immune Responses to AAV-Mediated Gene Transfer. J. Innate Immun. 2015, 7, 302–314.
- Manno, C.S.; Pierce, G.F.; Arruda, V.R.; Glader, B.; Ragni, M.; Rasko, J.J.; Rasko, J.; Ozelo, M.C.; Hoots, K.; Blatt, P.; et al. Successful Transduction of Liver in Hemophilia by AAV-Factor IX and Limitations Imposed by the Host Immune Response. Nat. Med. 2006, 12, 342–347.
- Nidetz, N.F.; McGee, M.C.; Tse, L.V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-Associated Viral Vector-Mediated Immune Responses: Understanding Barriers to Gene Delivery. Pharmacol. Ther. 2020, 207, 107453.
- Thran, M.; Mukherjee, J.; Pönisch, M.; Fiedler, K.; Thess, A.; Mui, B.L.; Hope, M.J.; Tam, Y.K.; Horscroft, N.; Heidenreich, R.; et al. MRNA Mediates Passive Vaccination against Infectious Agents, Toxins, and Tumors. EMBO Mol. Med. 2017, 9, 1434–1447.
- Van Hoecke, L.; Roose, K. How MRNA Therapeutics Are Entering the Monoclonal Antibody Field. J. Transl. Med. 2019, 17, 54.
- Pardi, N.; Secreto, A.J.; Shan, X.; Debonera, F.; Glover, J.; Yi, Y.; Muramatsu, H.; Ni, H.; Mui, B.L.; Tam, Y.K.; et al. Administration of Nucleoside-Modified MRNA Encoding Broadly Neutralizing Antibody Protects Humanized Mice from HIV-1 Challenge. Nat. Commun. 2017, 8, 14630.
- Deal, C.E.; Carfi, A.; Plante, O.J. Advancements in MRNA Encoded Antibodies for Passive Immunotherapy. Vaccines 2021, 9, 108.
- Schlake, T.; Thran, M.; Fiedler, K.; Heidenreich, R.; Petsch, B.; Fotin-Mleczek, M. MRNA: A Novel Avenue to Antibody Therapy? Mol. Ther. 2019, 27, 773–784.
