The surface of the eye is directly exposed to the external environment, protected only by a thin tear film, and may therefore be damaged by contact with ambient particulate matter, liquids, aerosols, or vapors. In the workplace or home, the eye is subject to accidental or incidental exposure to cleaning products and pesticides. Organic matter may enter the eye and cause infection. Ocular surface damage can trigger a range of symptoms such as itch, discharge, hyperemia, photophobia, blurred vision, and foreign body sensation. Toxin exposure can be assessed clinically in multiple ways, including via measurement of tear production, slit-lamp examination, corneal staining, and conjunctival staining. At the cellular level, environmental toxins can cause oxidative damage, apoptosis of corneal and conjunctival cells, cell senescence, and impaired motility. Outcomes range from transient and reversible with complete healing to severe and sight-compromising structural changes.
- eyes
- toxicity
- vision
- cornea
- pesticides
1. Introduction
2. Assessments of Ocular Toxicity
2.1. The Draize Eye Test
2.2. In Vitro Testing: Reconstructed Human Cornea-like Epithelium (RhCE)
2.3. In Silico Models
3. Pesticide Exposure
3.1. Pesticide Overview
Pesticides are potent environmental pollutants that are especially relevant to workers in the agricultural industry, exterminators, and pesticide manufacturers [111][40]. Approximately 866 million workers are employed in agriculture worldwide representing about 20% of the world’s wage-earning labor force, making occupational exposure to pesticides a pressing global health concern [112,113][41][42]. Pesticide use has increased steadily, and exposure is a health concern for the general population since phenomena such as pesticide drift or the presence of residues in food or drinking water can have deleterious health consequences [114,115][43][44]. The reporting of pesticide exposure-related health concerns is complicated by the varying levels of toxicity of different agro-chemicals, as well as the variability in exposure level and route of exposure (ingestion, inhalation, skin, or mucous membrane absorption) [116][45]. Pesticides, categorized as insecticides, herbicides, and fungicides, are often composed of organophosphates, organochlorines, and carbamate compounds [117,118,119,120][46][47][48][49]. These classes of compounds interact with several cellular receptors and interfere with normal bodily function. The health concerns related to pesticide exposure have been extensively documented, and chronic exposure to toxic pesticides has been linked to increased risk of cancer, dermatoses, and genotoxic, neurotoxic, and respiratory consequences [121,122,123][50][51][52]. Pesticide application leads to high levels of ocular exposure to toxic chemicals [124][53]. Pesticides can easily make their way into the eye from accidental splashing or by rubbing the eye with contaminated hands or cloths or by absorption from the air [125,126][54][55]. While exposure to pesticides is common, the impact of the ocular route of exposure and its consequences is poorly understood. Unfortunately, there is a gap in the medical literature regarding the effects of pesticides, especially pesticides of different classes, on the ocular surface.3.2. Herbicides and Insecticides
The herbicide paraquat, an organochlorine dipyridylium quaternary ammonium salt, is used frequently in agricultural fields and is known to be toxic to the ocular surface. Paraquat has been banned in European Union since 2007. Its toxicity is believed to relate to paraquat recycling in redox metabolism. Paraquat is an easily reducible organic cation, which interacts favorably with the reductive agent NADPH [127][56]. NADPH is a cellular electron carrier involved in many bio-reductive pathways for cellular metabolism and easily donates an electron to paraquat to become NADP+. This causes disruptions in cellular metabolism, as it depletes the NADPH pool of the cell and interrupts metabolic homeostasis. The depletion of NADPH also causes the accumulation of oxygen free radicals such as superoxide since these species are reduced by NADPH as a cytoprotective measure. The generation of free radicals causes tissue damage at the ocular surface due to the highly reactive nature of free radicals, which steal electrons from key biological molecules. On the ocular surface, a common result of free radical damage is conjunctivalization of the cornea with vascular pannus [127][56]. Severe injury may result in a chronically disordered ocular surface, manifesting in symptoms such as dryness, punctal stenosis, symblepharon, ankyloblpharon, forniceal shortening, entropion, and trichiasis [128,129][57][58]. Early appropriate treatment by flushing thoroughly with water may avoid highest levels of injury and minimize damage to minor corneal opacity and pannus as the main complications [130][59]. Paraquat-containing pesticide mixtures such as preeglox-L, which also contains diquat and surfactants, have also been linked to corneal epithelium deterioration [131][60]. Many herbicides contain the active ingredient glyphosate, an organophosphate compound that has toxic effects on several bodily systems. Organophosphates inhibit acetylcholinesterase (AChE), a key enzyme in the nervous system, by phosphorylating a serine hydroxyl group of its active site [132,133][61][62]. The inhibition of AChE by pesticides is known to cause eyelid muscle twitching, eye pain, and miosis [132,134][61][63]. Glyphosate has been shown to cause conjunctival irritation and superficial corneal injury, especially in cases where eye irrigation is delayed. [135,136][64][65]. Organophosphate exposure has also been linked to decreased glutathione content and increased levels of oxidative stress as measured by malondialdehyde levels in mouse eye and brain tissue upon exposure to the insecticide chlorpyrifos [137,138,139][66][67][68]. Cellular disruption via organophosphate pesticide exposure may result from inhibition of antioxidant enzymes such as superoxide dismutase and catalase, as well as an increase in inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β [140,141,142,143,144][69][70][71][72][73]. Flubendamide is a newer synthetic phthalic acid diamide insecticide with low immediate toxicity to humans [145][74]. The effects of flubendiamide on the ocular surface were studied in non-target Drosophila melanogaster to evaluate cross-reactivity in species at which the insecticide is not directed. It was found that flubendiamide altered the compound eye architecture and bristle pattern orientation in four generations of non-target D. melanogaster at doses consistent with those administered in fields in India [146,147][75][76]. The irritative nature of flubendiamide is further explored in a report published by the Food Safety Commission of Japan, as the insecticide was linked to ocular inflammation in rats [148][77].3.3. Fungicides
Mancozeb, a manganese/zinc ethylene-bis-dithiocarbamate fungicide, inhibits enzyme activity in fungi by complexing with enzymes containing sulfhydryl groups including those that participate in generation of ATP. This carbamate pesticide has been shown to cause toxic epidermal necrolysis and ocular lesions in cases of human exposure [149][78]. Carbamate pesticides, like organophosphate pesticides, are known to affect the AChE enzyme in human cells. Carbamates cause the carbamylation of AChE in neuronal synapses and neuromuscular junctions, and whereas organophosphates bind irreversibly to AChE, carbamates bind reversibly to the enzyme [150][79]. A study conducted at a seed supply warehouse in Japan identified n-butyl isocyanate, a hydrolyzed product of the fungicide benomyl as the cause for ocular irritation among several workers [151][80]. This finding has significant implications on regulatory measures for commercially used pesticides, as the safety of not only the pesticide must be taken into account but also the products of its degradation.4. Workplace Ocular Injuries
4.1. Overview
The workplace is a common site of ocular injuries, as approximately 2000 U.S. workers experience job-related eye injuries requiring medical treatment each day [152,153][81][82]. These injuries can be divided into three broad categories: striking or scraping, penetrating, and chemical and thermal burns [154,155,156][83][84][85]. Striking or scraping constitutes a common type of ocular injury, and involves the ejection of small particles such as dust, wood chips, or cement chips into the ocular surface, as well as larger objects that result in blunt trauma to the eye [157][86]. Penetration occurs when objects such as nails, staples, or slivers of wood or metal move through the surface of the eye and potentially result in the permanent loss of vision [158,159][87][88]. Chemical and thermal burns to the eye are frequently caused by industrial chemicals and cleaning products, and welding processes respectively [154][83]. A cross-sectional retrospective study used de-identified data from a large-scale employer survey of individuals reported to have ocular workplace injuries in the United States between 2011 and 2018 showed the highest likelihood of this type of injury in those employed in: fishing, farming and forestry; construction; and production industries [160][89].4.2. Foreign Object Injuries
In the fishing industry and in sports fishing, injury can occur when fishing hooks, lures, rod tips, or lines accidentally strike the eye [161,162,163,164][90][91][92][93]. Any eye structure may be involved with damage ranging from corneal abrasion to penetrating injury to globe rupture. Lenses, particularly wraparound lenses can protect the eye during fishing. Wood injuries may occur in forestry workers, wood workers, and gardeners [165][94]. Infections of bacterial or fungal origin are a significant risk, especially if the wood fragment is not removed promptly [166,167][95][96]. The high infection rate is attributed to the pores on the wood surface and the characteristics of organic and vegetative matter, which provide bacterial growth medium [168][97]. The infection may manifest as orbital cellulitis, abscess formation, and even intracranial infection. Detection of wood in the eye is challenging because it is carbon-containing and not visible on conventional x-ray may not image well on CT or MRI [169,170][98][99]. If the chip is small and on the surface, it may be flushed with eyewash; however, deeper penetration shards may require surgical intervention and antibiotic treatment [171][100]. Metal workers are particularly susceptible to dry eye according to a study by Ai et al. [172][101]. They attribute the vulnerability of metal workers to dry eye disease to their exposure to dust and chemicals. In a cross-sectional study of welders in Turkey, exposure to cadmium and lead were correlated with dry eye disease [173][102]. Chen et al. also found lead exposure and presence of lead in tears to be associated with dry eye disease [174][103]. Metallic foreign bodies can enter the eye during use of hammer and nail, nail gun, or stapler (Figure 1) [175,176,177,178][104][105][106][107]. Metallic foreign body removal is key in order to avoid consequences such as infection, swelling, inflammation, astigmatism, and opacification of the cornea [179][108]. Release of iron or copper from a retained foreign body in the eye can lead to cataracts, glaucoma, and pigment changes on the retina [180,181,182][109][110][111].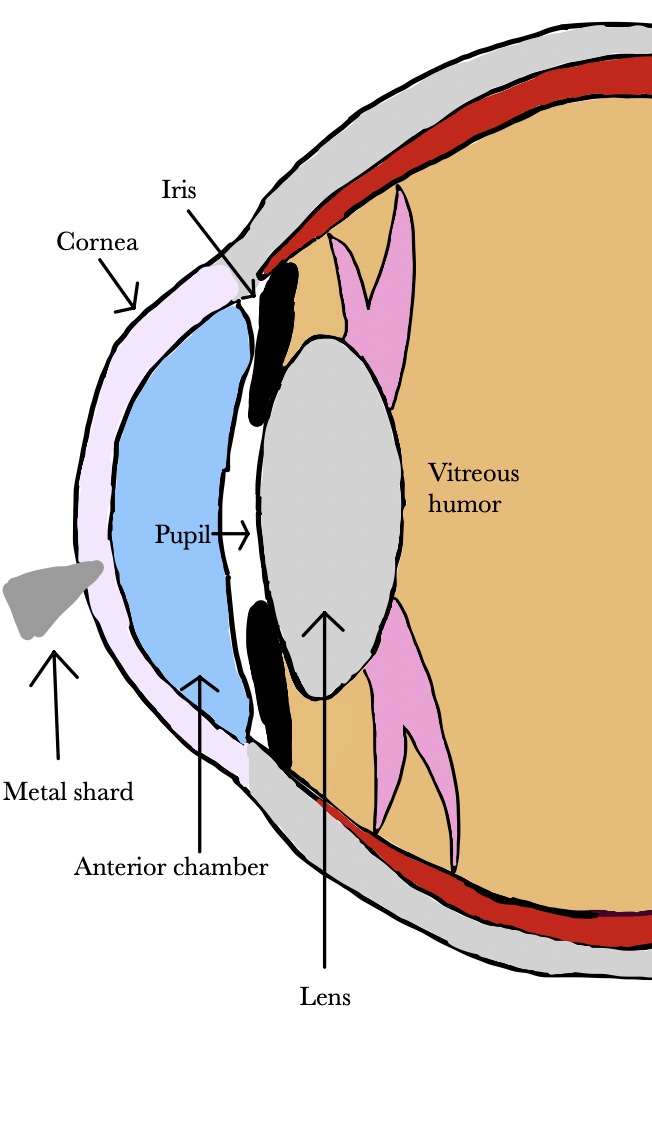

4.3. Chemical Injuries
Cleaning products used around the home and office are often formulated with chemicals that can damage the eye. Chemical burns to the eye can come from acids, alkalis, or alcohol [183][112]. Acids cause protein coagulation, which somewhat limits damage by forming a self-containing barrier while alkalis are lipophilic, cause saponification and penetrate more deeply into tissue, leading to extensive and severe damage to the cornea [184,185][113][114]. Alkali burns can result in loss of limbal epithelial stem cells that are essential for regeneration of corneal epithelium [186][115]. In the United States, bleaches, categorized as alkali, accounted for more than 25% of ocular exposures reported to poison control centers between January 2000 and December 2016 [187][116]. Bleach can cause burning sensation, tearing, photophobia, and conjunctival abrasions [188,189,190][117][118][119]. Hydrofluoric acid is a highly reactive compound used in industry and some cleaning and rust-removing products. It can cause burns, tearing, conjunctivitis, and corneal ulcers and opacification [191,192][120][121]. Exposure of the eye to ethanol, which is often used as a disinfectant, can damage corneal epithelial and stromal cells, and cause inflammation and proinflammatory cytokine release [193,194][122][123].4.4. Preventing Damage from Chemicals and Foreign Bodies
Particles in the eye and chemical eye burns require immediate flushing and therefore access to water or other rinsing solutions in the workplace is essential [195][124]. Most occupational eye injuries are potentially preventable [196][125]. Eye protection needs to fully cover the eyes [197][126]. There are multiple forms of appropriate eye protection, some of which include goggles, face shields, and full-face respirators that reduce the likelihood of work-related eye injuries [191,198,199,200][120][127][128][129]. Indirectly vented goggles that fit from the corners of the eye across the brow provide effective protection from splashes, sprays, and respiratory droplets that may be encountered in the workplace [156][85]. Although goggles are viable in shielding the eyes from irritants, other parts of the face are neglected by goggles and thus remain vulnerable despite goggle usage. Face shields that wrap around the face to the ears can be utilized in addition to goggles to provide increased protection from splashes and sprays for the entire face as opposed to simply the eyes. Requiring these forms of protection in the workplace can contribute to a reduction in daily work-related ocular injuries [201,202][130][131].5. Dry Eye Disease and its Consequences
Dry eyes and external ocular surface disease are thoroughly addressed in the ophthalmology literature[1]. Different types of dry eye syndromes are described. There are many prescription and non prescription medications available for treating the symptoms of dry eyes[2] .
Yet, the external environmental factors causing dry eyes are only briefly discussed. After 9/11, many first responders and workers were exposed to toxic dust[3]. There was extensive research on cancer, which was the highest priority, but many of those exposed are suffering from the chronic symptoms of dryness, burning, redness, irritation and light sensitivity even decades later[4]. A parallel situation of severe health consequences overshadowing ophthalmic issues resulting from exposure to the burn pits in Iraq[5]. The soldiers and civilian workers may experience dry eyes, but research focuses on higher priority sequelae[6]. Even the recent Ohio train derailment demonstrates the danger of toxic chemicals to nearby residents. Lawsuits are being pursue, but ophthalmologists are not being called upon to describe the short or long-term consequences. Artificial tears are expensive and bring danger of infection[7]. It is hard to spend hours working at a computer and conducting everyday activities vital to functioning in the modern world when one’s eye are burning.
This paper begins to address these issues in an exhaustive scientific fashion.
References
- Rege A, Kulkarni V, Puthran N, Khandgave T. A Clinical Study of Subtype-based Prevalence of Dry Eye. J Clin Diagn Res 2013, 7, 2207-2210, 10.7860/JCDR/2013/6089.3472.Fischer, I.; Milton, C.; Wallace, H. Toxicity testing is evolving! Toxicol. Res. 2020, 9, 67–80.
- Kumari N, Kusumesh R, Kumari R, Sinha BP, Singh V. Comparative evaluation of effectiveness of twenty versus fifty percent autologous serum eye drops in treatment of dry eye. . Indian J Ophthalmol. 2023, 71, 1603-1607, 10.4103/IJO.IJO_2684_22.Chuprina, A.; Lukin, O.; Demoiseaux, R.; Buzko, A.; Shivanyuk, A. Drug- and lead-likeness, target class, and molecular diversity analysis of 7.9 million commercially available organic compounds provided by 29 suppliers. J. Chem. Inf. Model. 2010, 50, 470–479.
- Mears MJ, Aslaner DM, Barson CT, Cohen MD, Gorr MW, Wold LE. Health effects following exposure to dust from the World Trade Center disaster: An update. . Life Sci. 2022, 289, 120147, 10.1016/j.lfs.2021.120147.Haring, R.S.; Sheffield, I.D.; Channa, R.; Canner, J.K.; Schneider, E.B. Epidemiologic Trends of Chemical Ocular Burns in the United States. JAMA Ophthalmol. 2016, 134, 1119–1124.
- Singh A, Zeig-Owens R, Cannon M, et al. All-cause and cause-specific mortality in a cohort of WTC-exposed and non-WTC-exposed firefighters. Occup Environ Med. 2023, 2022, 108703, 10.1136/oemed-2022-108703.Prior, H.; Casey, W.; Kimber, I.; Whelan, M.; Sewell, F. Reflections on the Progress towards Non-Animal Methods for Acute Toxicity Testing of Chemicals. Regul. Toxicol. Pharmacol. 2019, 102, 30–33.
- Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. . Part Fibre Toxicol. 2021, 18, 45, 10.1186/s12989-021-00435-w .Fitzhugh, O.G.; Woodard, G. The toxicities of compounds related to 2,3-dimercaptopropanol (BAL) with a note on their relative therapeutic efficiency. J. Pharmacol. Exp. Ther. 1946, 87, 23–27.
- Modi, Y. S., Qurban, Q., Zlotcavitch, L., Echeverri, R. J., Feuer, W., Florez, H., Galor, A. Ocular surface symptoms in veterans returning from operation Iraqi freedom and operation enduring freedom. . Investigative ophthalmology & visual science 2014, 55, 650–653, 10.1167/iovs.13-13330.Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390.
- CDC sounds alarm over eye drops linked to dozens of infections, 1 death . Ophthalmology Times. Retrieved 2023-4-12Wilhelmus, K.R. The Draize eye test. Surv. Ophthalmol. 2001, 45, 493–515.
- Barile, F.A. Validating and troubleshooting ocular in vitro toxicology tests. J. Pharm. Toxicol. Methods 2010, 61, 136–145.
- Vinardell, M.P.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59.
- Lieto, K.; Skopek, R.; Lewicka, A.; Stelmasiak, M.; Klimaszewska, E.; Zelent, A.; Szymański, Ł.; Lewicki, S. Looking into the Eyes-In Vitro Models for Ocular Research. Int. J. Mol. Sci. 2022, 23, 9158.
- Curren, R.D.; Harbell, J.W. Ocular safety: A silent (in vitro) success story. Altern. Lab. Anim. 2002, 30, 69–74.
- Bonneau, N.; Baudouin, C.; Réaux-Le Goazigo, A.; Brignole-Baudouin, F. An overview of current alternative models in the context of ocular surface toxicity. J. Appl. Toxicol. 2022, 42, 718–737.
- Chacón, M.; Vázquez, N.; Persinal-Medina, M.; Alonso-Alonso, S.; Alcalde, I.; Merayo-Lloves, J.; Meana, Á. In-house performance assessment of 3D QobuR-Reconstructed Human Cornea-Like Epithelium (RhCE) for the evaluation of eye hazard. Toxicol. In Vitro 2022, 82, 105390.
- Narda, M.; Ramos-Lopez, D.; Mun, G.; Valderas-Martinez, P.; Granger, C. Three-tier testing approach for optimal ocular tolerance sunscreen. Cutan. Ocul. Toxicol. 2019, 38, 212–220.
- Matsuda, S.; Hisama, M.; Shibayama, H.; Itou, N.; Iwaki, M. Application of the reconstructed rabbit corneal epithelium model to assess the in-vitro eye irritant test of chemicals. Yakugaku Zasshi 2009, 129, 1113–1120.
- Kaluzhny, Y.; Kandárová, H.; Hayden, P.; Kubilus, J.; d’Argembeau-Thornton, L.; Klausner, M. Development of the EpiOcular(TM) eye irritation test for hazard identification and labelling of eye irritating chemicals in response to the requirements of the EU cosmetics directive and REACH legislation. Altern. Lab. Anim. 2011, 39, 339–364.
- Alépée, N.; Leblanc, V.; Adriaens, E.; Grandidier, M.H.; Lelièvre, D.; Meloni, M.; Nardelli, L.; Roper, C.S.; Santirocco, E.; Toner, F.; et al. Multi-laboratory validation of SkinEthic HCE test method for testing serious eye damage/eye irritation using liquid chemicals. Toxicol. In Vitro 2016, 31, 43–53.
- Stern, M.; Klausner, M.; Alvarado, R.; Renskers, K.; Dickens, M. Evaluation of the EpiOcular((TM)) tissue model as an alternative to the Draize eye irritation test. Toxicol. In Vitro 1998, 12, 455–461.
- Kandarova, H.; Letasiova, S.; Adriaens, E.; Guest, R.; Willoughby, J.A., Sr.; Drzewiecka, A.; Gruszka, K.; Alépée, N.; Verstraelen, S.; Van Rompay, A.R. CON4EI: EpiOcular™ Eye Irritation Test (EpiOcular™ EIT) for hazard identification and labelling of eye irritating chemicals. Toxicol. In Vitro 2018, 49, 21–33.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63.
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69.
- Cotovio, J.; Grandidier, M.H.; Portes, P.; Roguet, R.; Rubinstenn, G. The in vitro skin irritation of chemicals: Optimisation of the EPISKIN prediction model within the framework of the ECVAM validation process. Altern. Lab. Anim. 2005, 33, 329–349.
- Ichijima, H.; Ohashi, J.; Cavanagh, H.D. Effect of contact-lens-induced hypoxia on lactate dehydrogenase activity and isozyme in rabbit cornea. Cornea 1992, 11, 108–113.
- Maurer, J.K.; Parker, R.D.; Jester, J.V. Extent of initial corneal injury as the mechanistic basis for ocular irritation: Key findings and recommendations for the development of alternative assays. Regul. Toxicol. Pharm. 2002, 36, 106–117.
- Eskes, C.; Bessou, S.; Bruner, L.; Curren, R.; Harbell, J.; Jones, P.; Kreiling, R.; Liebsch, M.; McNamee, P.; Pape, W.; et al. Eye Irritation. Altern. Lab. Anim. 2005, 33, 47–81.
- Lebrun, S.; Nguyen, L.; Chavez, S.; Chan, R.; Le, D.; Nguyen, M.; Jester, J.V. Same-chemical comparison of nonanimal eye irritation test methods: Bovine corneal opacity and permeability, EpiOcular™, isolated chicken eye, ocular Irritection®, OptiSafe™, and short time exposure. Toxicol. In Vitro 2021, 72, 105070.
- Doucet, O.; Lanvin, M.; Thillou, C.; Linossier, C.; Pupat, C.; Merlin, B.; Zastrow, L. Reconstituted human corneal epithelium: A new alternative to the Draize eye test for the assessment of the eye irritation potential of chemicals and cosmetic products. Toxicol. In Vitro 2006, 20, 499–512.
- Abbate, I.; Zappulla, C.; Santonocito, M.; Viola, S.; La Rosa, L.R.; De Pasquale, G.; Caviola, E.; Meloni, M.; Curatolo, M.C.; Mazzone, M.G. Preclinical study of a new matrix to help the ocular surface in dry eye disease. Exp. Eye Res. 2022, 222, 109168.
- Leblanc, V.; Yokota, M.; Grandidier, M.H.; Yoshida, D.; Adriaens, E.; Cotovio, J.; Kyoutani, D.; Alépée, N. SkinEthic™ HCE Eye Irritation Test: Similar performance demonstrated after long distance shipment and extended storage conditions. Toxicol. In Vitro 2019, 54, 202–214.
- Alépée, N.; Grandidier, M.H.; Teluob, S.; Amaral, F.; Caviola, E.; De Servi, B.; Martin, S.; Meloni, M.; Nardelli, L.; Pasdelou, C.; et al. Validation of the SkinEthic HCE Time-to-Toxicity test method for eye hazard classification of chemicals according to UN GHS. Toxicol. In Vitro 2022, 80, 105319.
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS); United Nations: New York, NY, USA; Geneva, Switzerland, 2019; Available online: https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev08/ST-SG-AC10-30-Rev8e.pdf (accessed on 5 January 2023).
- Alépée, N.; Leblanc, V.; Grandidier, M.H.; Teluob, S.; Viricel, A.; Adriaens, E.; Michaut, V. SkinEthic HCE Time-to-Toxicity on solids: A test method for distinguishing chemicals inducing serious eye damage, eye irritation and not requiring classification and labelling. Toxicol. In Vitro 2021, 75, 105203.
- Deeb, O.; Goodarzi, M. In silico quantitative structure toxicity relationship of chemical compounds: Some case studies. Curr. Drug Saf. 2012, 7, 289–297.
- Valerio, L.G., Jr. In silico toxicology for the pharmaceutical sciences. Toxicol. Appl. Pharmacol. 2009, 241, 356–370.
- Valerio, L.G., Jr. In silico toxicology models and databases as FDA Critical Path Initiative toolkits. Hum. Genom. 2011, 5, 200–207.
- Sinha, M.; Dhawan, A.; Parthasarathi, R. In silico approaches in predictive genetic toxicology. Methods Mol. Biol. 2019, 2031, 351–373.
- Rim, K.T. In silico prediction of toxicity and its applications for chemicals at work. Toxicol. Environ. Health Sci. 2020, 12, 191–202.
- Chinen, K.; Malloy, T. QSAR Use in REACH analyses of alternatives to predict human health and environmental toxicity of alternative chemical substances. Integr. Environ. Assess. Manag. 2020, 16, 745–760.
- Fourches, D.; Muratov, E.; Tropsha, A. Trust, but verify: On the importance of chemical structure curation in cheminformatics and QSAR modeling research. J. Chem. Inf. Model. 2010, 50, 1189–1203.
- Maroni, M.; Fait, A.; Colosio, C. Risk assessment and management of occupational exposure to pesticides. Toxicol. Lett. 1999, 107, 145–153.
- Food and Agriculture Organization of the United Nations. World Food and Agriculture—Statistical Yearbook 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022.
- Trask, C.; Khan, M.I.; Adebayo, O.; Boden, C.; Bath, B. Equity in whom gets studied: A systematic review examining geographical region, gender, commodity, and employment context in research of low back disorders in farmers. Agromedicine 2015, 20, 273–281.
- Bish, M.; Oseland, E.; Bradley, K. Off-target pesticide movement: A review of our current understanding of drift due to inversions and secondary movement. Weed Technol. 2021, 35, 345–356.
- Cech, R.; Zaller, J.G.; Lyssimachou, A.; Clausing, P.; Hertoge, K.; Linhart, C. Pesticide drift mitigation measures appear to reduce contamination of non-agricultural areas, but hazards to humans and the environment remain. Sci. Total Environ. 2022, 854, 158814.
- Sanyal, S.; Das, P.; Law, S. Effect of chronic pesticide exposure on murine cornea: A histopathological, cytological and flow cytometric approach to study ocular damage by xenobiotics. Cell Biol. Toxicol. 2016, 32, 7–22.
- Alozi, M.; Rawas-Qalaji, M. Treating organophosphates poisoning: Management challenges and potential solutions. Crit. Rev. Toxicol. 2020, 50, 764–779.
- Coats, J.R. Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ. Health Perspect. 1990, 87, 255–262.
- Jayara, J.R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100.
- Hou, C.; Wang, Z.; Li, X.; Bai, Y.; Chai, J.; Li, X.; Gao, J.; Xu, H. Study of modeling and optimization for predicting the acute toxicity of carbamate pesticides using the binding information with carrier protein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 273, 121038.
- Wesseling, C.; Aragón, A.; Castillo, L.; Corriols, M.; Chaverri, F.; de la Cruz, E.; Keifer, M.; Monge, P.; Partanen, T.J.; Ruepert, C.; et al. Hazardous pesticides in Central America. Int. J. Occup. Environ. Health 2001, 7, 287–294.
- Mamane, A.; Baldi, I.; Tessier, J.F.; Raherison, C.; Bouvier, G. Occupational exposure to pesticides and respiratory health. Eur. Respir. Rev. 2015, 24, 306–319.
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12.
- Clippinger, A.J.; Raabe, H.A.; Allen, D.G.; Choksi, N.Y.; van der Zalm, A.J.; Kleinstreuer, N.C.; Barroso, J.; Lowit, A.B. Human-relevant approaches to assess eye corrosion/irritation potential of agrochemical formulations. Cutan. Ocul. Toxicol. 2021, 40, 145–167.
- Fareed, M.; Kesavachandran, C.N.; Pathak, M.K.; Bihari, V.; Kuddus, M.; Srivastava, A.K. Visual disturbances with cholinesterase depletion due to exposure of agricultural pesticides among farm workers. Toxicol. Environ. Chem. 2012, 94, 1601–1609.
- Lu, J.L. Acute pesticide poisoning among cut-flower farmers. J. Environ. Health 2007, 70, 38–43.
- McKeag, D.; Maini, R.; Taylor, H.R. The ocular surface toxicity of paraquat. Br. J. Ophthalmol. 2002, 86, 350–351.
- Joyce, M. Ocular damage caused by paraquat. Br. J. Ophthalmol. 1969, 53, 688–690.
- Vlahos, K.; Goggin, M.; Coster, D. Paraquat causes chronic ocular surface toxicity. Aust. N. Z. J. Ophthalmol. 1993, 21, 187–190.
- Jian-Wei, L.; Xiu-Yun, L.; Ai-Jun, D. Effectiveness of heparin eye drops in paraquat-induced ocular injury. Cutan. Ocul. Toxicol. 2017, 36, 377–380.
- Nirei, M.; Hayasaka, S.; Nagata, M.; Tamai, A.; Tawara, T. Ocular injury caused by Preeglox-L, a herbicide containing paraquat, diquat and surfactants. Jpn. J. Ophthalmol. 1993, 37, 43–46.
- Vale, A.; Lotti, M. Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015, 131, 149–168.
- Ganie, S.Y.; Javaid, D.; Hajam, Y.A.; Reshi, M.S. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: A critical appraisal. Toxicology 2022, 472, 153181.
- Amend, N.; Langgartner, J.; Siegert, M.; Kranawetvogl, T.; Koller, M.; John, H.; Pflügler, C.; Mögele-Schmid, C.; Worek, F.; Thiermann, H.; et al. A case report of cholinesterase inhibitor poisoning: Cholinesterase activities and analytical methods for diagnosis and clinical decision making. Arch. Toxicol. 2020, 94, 2239–2247.
- Bradberry, S.M.; Proudfoot, A.T.; Vale, J.A. Glyphosate poisoning. Toxicol. Rev. 2004, 23, 159–167.
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Castellano, V.J.; Martínez, M.; Martin, M.T.; Nozal, M.J.; Bernal, J.L. Toxicokinetics of glyphosate and its metabolite aminmethyl phosphonic acid in rats. Toxicol. Lett. 2009, 190, 91–95.
- Ma, P.; Wu, Y.; Zeng, Q.; Gan, Y.; Chen, J.; Ye, X.; Yang, X. Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem. Toxicol. 2013, 58, 177–183.
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Daoud, A.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. Int. 2020, 27, 11663–11670.
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138.
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E. Antioxidant and anti-inflammatory potential of thymoquinone and lycopene mitigate the chlorpyrifos-induced toxic neuropathy. Pharmaceuticals 2021, 14, 940.
- Hernández, A.F.; Lacasaña, M.; Gil, F.; Rodríguez-Barranco, M.; Pla, A.; López-Guarnido, O. Evaluation of pesticide-induced oxidative stress from a gene-environment interaction perspective. Toxicology 2013, 307, 95–102.
- Banks, C.N.; Lein, P.J. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 2012, 33, 575–584.
- Goswami, D.G.; Kant, R.; Ammar, D.A.; Agarwal, C.; Gomez, J.; Agarwal, R.; Saba, L.M.; Fritz, K.S.; Tewari-Singh, N. Toxic consequences and oxidative protein carbonylation from chloropicrin exposure in human corneal epithelial cells. Toxicol. Lett. 2020, 322, 1–11.
- Medithi, S.; Kasa, Y.D.; Kankipati, V.R.; Kodali, V.; Jee, B.; Jonnalagadda, P.R. Impact of micronutrient supplementation on pesticide residual, acetylcholinesterase activity, and oxidative stress among farm children exposed to pesticides. Front. Public Health 2022, 10, 872125.
- Samurkas, A.; Yao, L.; Hadiatullah, H.; Ma, R.; Xie, Y.; Sundarraj, R.; Zuilhof, H.Z. Ryanodine receptor as insecticide target. Curr. Pharm. Des. 2022, 28, 26–35.
- Sarkar, S.; Roy, S. Flubendiamide induces transgenerational compound eye alterations in Drosophila melanogaster. Interdiscip. Toxicol. 2017, 10, 142–147.
- Sarkar, S.; Roy, A.; Roy, S. Flubendiamide affects visual and locomotory activities of Drosophila melanogaster for three successive generations (P, F1 and F2). Invert. Neurosci. 2018, 18, 6.
- Food Safety Commission of Japan. Flubenziamide (Pesticides). Food Saf. 2019, 7, 15–19.
- Zakharov, S.; Csomor, J.; Urbanek, P.; Pelclova, D. Toxic epidermal necrolysis after exposure to dithiocarbamate fungicide Mancozeb. Basic Clin. Pharmacol Toxicol. 2016, 118, 87–91.
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335.
- Matsukawa, T.; Yokoyama, K.; Itoh, H. Ocular irritation from product of pesticide degradation among workers in a seed warehouse. Ind. Health 2015, 53, 95–99.
- Forrest, K.Y.; Cali, J.M. Epidemiology of lifetime work-related eye injuries in the U.S. population associated with one or more lost days of work. Ophthalmic Epidemiol. 2009, 16, 156–162.
- Kyriakaki, E.D.; Symvoulakis, E.K.; Chlouverakis, G.; Detorakis, E.T. Causes, occupational risk and socio-economic determinants of eye injuries: A literature review. Med. Pharm. Rep. 2021, 94, 131–144.
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019.
- Makwana, T.; Gupta, N.; Vashist, P. Ocular emergencies in the South Asia region. Community Eye Health 2019, 31, S1–S4.
- Peate, W.F. Work-related eye injuries and illnesses. Am. Fam. Physician 2007, 75, 1017–1022.
- McGwin, G.; Owsley, C. Incidence of emergency-department–treated eye injury in the United States. Arch. Ophthalmol. 2005, 123, 662–666.
- Adriono, G.A.; Agustiawan, R.; Fibrian, K.C.; Ardiani, L.S.; Irawati, Y. Variations in clinical manifestations and outcomes of penetrating ocular injuries with intraocular foreign bodies: A case series. J. Surg. Case Rep. 2022, 2022, rjac198.
- Khanam, S.; Agarwal, A.; Goel, R.; Rathie, N.; Raut, A.; Raghav, S.; Kumar, S.; Chhabra, M.; Singh, S.; Kumar, S. Clinical presentation and management strategies in intraorbital foreign bodies. Case Rep. Ophthalmol. Med. 2021, 2021, 6645952.
- Hom, G.L.; Kalurm, A.; Iyer, A.; Singh, R.P. Ocular occupational injuries in the United States between 2011–2018. Occup. Med. 2022, 72, 255–259.
- Awan, A.; Scott, J.A. Corneal injury from a fishing line: A new mechanism. Eye 2006, 20, 1084–1086.
- Ono, T.; Takahashi, S.; Mori, Y.; Nejimar, R.; Iwasaki, T.; Kataoka, Y.; Miyai, T.; Miyata, K. 1Severe fishhook-related ocular injury: A case series. Trauma Case Rep. 2021, 37, 100574.
- Choovuthayakorn, J.; Chavengsaksongkram, P.; Watanachai, N.; Chaidaroon, W. Penetrating eyelid and ocular fishhook-related injury. Case Rep. Ophthalmol. 2019, 24, 41–46.
- Purtskhvanidze, K.; Saeger, M.; Treumer, F.; Nölle, B.; Roider, J. Open globe and penetrating eyelid injuries from fish hooks. BMC Ophthalmol. 2019, 19, 26.
- Haavisto, A.K.; Sahraravand, A.; Puska, P.; Leivo, T. Eye injuries caused by wooden projectiles in Finland. Wilderness Environ. Med. 2022, 33, 284–289.
- Fulcher, T.P.; McNab, A.A.; Sullivan, T.J. Clinical features and management of intraorbital foreign bodies. Ophthalmology 2002, 109, 494–500.
- Al-Mujaini, A.; Al-Senawi, R.; Ganesh, A.; Al-Zuhaibi, S.; Al-Dhuhli, H. Intraorbital foreign body: Clinical presentation, radiological appearance and management. Sultan Qaboos Univ. Med. J. 2008, 8, 69–74.
- Hua, L.; Doll, T. A series of 3 cases of corneal abrasion with multiple etiologies. Optometry 2010, 81, 83–85.
- You, Y.Y.; Shi, B.J.; Wang, X.Y.; Chen, J.; Wang, Z.R.; Wang, X.H.; Jiang, F.G. Intraorbital wooden foreign bodies: Case series and literature review. Int. J. Ophthalmol. 2021, 14, 1619–1627.
- Pandit, K.; Sitaula, S.; Shrestha, G.B.; Joshi, S.N.; Chaudhary, M. Management of unusual missed diagnosis of a intra-orbital wooden foreign body: A case report and review of literature. Ann. Med. Surg. 2022, 79, 104017.
- Li, J.; Zhou, L.P.; Jin, J.; Yuan, H.F. Clinical diagnosis and treatment of intraorbital wooden foreign bodies. Chin. J. Traumatol. 2016, 19, 322–325.
- Ay, İ.E.; Demirezen, M.; Şenol, Y.; Til, A. Ocular health among industrial workers: A prevalence study of foreign body injury, refractive error, dry eye, pterygium and pingueculae. Med. Lav. 2022, 113, e2022044.
- Liou, Y.H.; Chen, Y.J.; Chen, W.L.; Li, K.Y.; Chou, Y.; Huang, Y.C.; Wang, C.C.; Lai, C.H. Associations between biomarkers of metal exposure and dry eye metrics in shipyard welders: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 17, 2264.
- Chen, Y.J.; Chen, Y.Y.; Lai, C.H. Clinical association between trace elements of tear and dry eye metrics. Sci. Rep. 2022, 12, 18052.
- Bouirig, K.; Cherkaoui, O. Iron deposition from a retained intraocular foreign body. N. Engl. J. Med. 2022, 387, e49.
- Khanduja, S.; Khurana, A.; Sachdeva, S.; Rathi, A.; Khurana, A.K. Tractor nail as impacted foreign body: Rare case scenario. Int. Ophthalmol. 2013, 33, 291–293.
- Irving Enrique, C.S.; Dhariana, A.R.; Vidal, S.V.; Carlos Felipe, P.H.; Lorena, W.G.; Gerardo, G.A. Conservative management of penetrating ocular trauma caused by a nail gun. Am. J. Ophthalmol. Case Rep. 2018, 11, 115–118.
- Burger, B.M.; Kelty, P.J.; Bowie, E.M. Ocular nail gun injuries: Epidemiology and visual outcomes. J. Trauma 2009, 67, 1320–1322.
- Elahi, S.; Saad, A.; Gatinel, D. Descemet membrane endothelial keratoplasty for corneal decompensation due to migrating metallic intracorneal foreign bodies in an aphakic eye following a 39-year-old blast injury: A case report. Am. J. Ophthalmol. Case Rep. 2021, 23, 101162.
- Al-Dwairi, R.; Msallam, M. Unilateral ocular siderosis bulbi due to missed metallic intraocular foreign body masquerading as anisocoria of neurological origin: A case report. Am. J. Case. Rep. 2021, 22, e930504.
- Doctor, M.B.; Parameswarappa, D.C.; Vaddavalli, P.K.; Rani, P.K. Intralenticular copper foreign body. BMJ Case Rep. 2020, 13, e240757.
- Ramakrishnan, T.; Constantinou, M.; Jhanji, V.; Vajpayee, R.B. Corneal metallic foreign body injuries due to suboptimal ocular protection. Arch. Environ. Occup. Health 2012, 67, 48–50.
- Said, D.; Harminder, D. Chemical burns acid or alkali, what’s the difference? Eye 2020, 34, 1299–1300.
- Al-Ghadeer, H.; Al Amry, M.; Aldihan, K.A.; Alobaidan, O.S.; AlQahtani, G.M.S.; Khandekar, R. Demographic, clinical profile and management outcomes of ocular chemical injuries in Saudi children. Clin. Ophthalmol. 2022, 16, 3247–3255.
- Bizrah, M.; Yusuf, A.; Ahmad, S. An update on chemical eye burns. Eye 2019, 33, 1362–1377.
- Tuft, S.J.; Shortt, A.J. Surgical rehabilitation following severe ocular burns. Eye 2009, 23, 1966–1971.
- Kamboj, A.; Spiller, H.A.; Casavant, M.J.; Kistamgari, S.; Chounthirath, T.; Smith, G.A. Household cleaning product-related ocular exposures reported to the United States poison control centres. Eye 2020, 34, 1631–1639.
- Slaughter, R.J.; Watts, M.; Vale, J.A.; Grieve, J.R.; Schep, L.J. The clinical toxicology of sodium hypochlorite. Clin. Toxicol. 2019, 57, 303–311.
- Tredici, C.; Fasciani, R.; Villano, A.; Gambini, G.; Caporossi, A. Efficacy of eye drops containing crosslinked hyaluronic acid and CoQ10 in restoring ocular health exposed to chlorinated water. Eur. J. Ophthalmol. 2020, 30, 430–438.
- Blackburn, J.; Levitan, E.B.; MacLennan, P.A.; Owsley, C.; McGwin, G., Jr. The epidemiology of chemical eye injuries. Curr. Eye Res. 2012, 37, 787–793.
- Bajraktarova-Valjakova, E.; Korunoska-Stevkovska, V.; Georgieva, S.; Ivanovski, K.; Bajraktarova-Misevska, C.; Mijoska, A.; Grozdanov, A. Hydrofluoric acid: Burns and systemic toxicity, protective measures, immediate and hospital medical treatment. Open Access Maced. J. Med. Sci. 2018, 6, 2257–2269.
- Atley, K.; Ridyard, E. Treatment of hydrofluoric acid exposure to the eye. Int. J. Ophthalmol. 2015, 8, 157–161.
- Lee, J.; Jun, J.H. Ocular chemical burn associated with gel type alcohol-based hand sanitizer: A case report. Medicine 2021, 100, e27292.
- Oh, J.Y.; Yu, J.M.; Ko, J.H. Analysis of ethanol effects on corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3852–3856.
- Claassen, K.; Rodil Dos Anjos, D.; Broding, H.C. Current status of emergency treatment of chemical eye burns in workplaces. Int. J. Ophthalmol. 2021, 14, 306–309.
- Lipscomb, H.J. Effectiveness of interventions to prevent work-related eye injuries. Am. J. Prev. Med. 2000, 18, 27–32.
- Balkhyour, M.A.; Ahmad, I.; Rehan, M. Assessment of personal protective equipment use and occupational exposures in small industries in Jeddah: Health implications for workers. Saudi J. Biol. Sci. 2019, 26, 653–659.
- Dain, S.J.; Huang, R.; Tiao, A.; Chou, B.R. When is protection from impact needed for the face as well as the eyes in occupational environments? Clin. Exp. Optom. 2018, 101, 392–396.
- Abu, E.K.; Ocansey, S.; Gyamfi, J.A.; Ntodie, M.; Morny, E.K. Epidemiology and visual outcomes of ocular injuries in a low resource country. Afr. Health Sci. 2020, 20, 779–788.
- Ahmed, F.; House, R.J.; Feldman, B.H. Corneal abrasions and corneal foreign bodies. Prim. Care 2015, 42, 363–375.
- Monaghan, P.F.; Bryant, C.A.; McDermott, R.J.; Forst, L.S.; Luque, J.S.; Contreras, R.B. Adoption of safety eyewear among citrus harvesters in rural Florida. J. Immigr. Minor. Health 2012, 14, 460–466.
- Sun, F.; Zhou, Y.; Dong, L.; Qin, H. Relationship between the use and type of eye protection and work-related corneal and conjunctival foreign body injuries. Inj. Prev. 2021, 27, 521–526.
