Phloem sap transport is essential for plant nutrition and development since it mediates redistribution of nutrients, metabolites and signaling molecules. However, its biochemical composition is not so well-known because phloem sap sampling is difficult and does not always allow extensive chemical analysis. EIn the past years, efforts have been devoted to metabolomics analyses of phloem sap using either liquid chromatography or gas chromatography coupled with mass spectrometry. Phloem sap metabolomics is of importance to understand how metabolites can be exchanged between plant organs and how metabolite allocation may impact plant growth and development.
This writing is an excerpt from Broussard et al. 2023.
- phloem
- sap
- metabolome
- metabolic cycle
1. Introduction
2. Phloem Sap Metabolites
Metabolic analyses carried out in the past decades with different techniques have shown that phloem sap is not just a concentrated sucrose/amino acid solution. In fact, many compounds of different metabolite families have been found, suggesting that phloem sap has metabolic functions beyond C, N and S redistribution from source leaves to sink organs. Nevertheless, it must be recognized that metabolites found in phloem sap depend on the analytical technique (typically, HPLC, GC-MS or LC-MS), and thus our current knowledge might not be strictly representative. Methods coupled to mass spectrometry allow analyses of many more metabolites than just HPLC, and thus GC-MS and LC-MS usually give access to a more representative picture of the metabolome. That said, GC-MS is usually dedicated to primary metabolites while LC-MS is more adapted to metabolites of higher molecular mass (such as secondary metabolites). In terms of concentration, current metabolomics techniques (such as GC-MS and LC-MS) are semi-quantitative and adapted to comparing samples rather than providing absolute concentrations (in mM). In general, sugars are the major component of phloem sap, representing more than 70% of phloem sap metabolites. In herbaceous plants, sucrose concentrations range from 400 to 1400 mM [14], with some variation (maize (Zea mays) 900 mM, [15]; 844 mM, [16]; rice (Oryza sativa) 574 mM, [17]; barley (Hordeum vulgare) 1030 mM, [18]; wheat (Triticum aestivum) 251 mM, [19]; and castor bean (Ricinus communis) 270 mM, [20]). Likewise, sucrose concentration varies considerably between tree species from 65 mM to 1 M (for instance, oak (Quercus robur) ~1 M, [21]; beech (Fagus sylvatica) 790 mM, [22]; magnolia (Magnolia kobus) 850 mM, [22]; Eucalyptus (Eucalyptus globulus) 220 mM, [23]; and lemon tree (Citrus limon) 65 mM, [24]). It is believed that other soluble sugars such as fructose and glucose are also present in phloem sap, and sometimes at relatively high concentrations. Evidence for the occurrence of hexoses has been provided with reliable phloem sap sampling techniques, including stylectomy [18][21][25][26], the EDTA method [26][27][28][29][30][31], the incision method [32][33] and centrifugation [34]. Fructose and glucose content depends on species (e.g., found in lemon tree, wheat and maize [27][28] but absent in castor bean [20][35]) and developmental stage; for instance, it depends on grain filling stage, N fertilization and CO2 concentration in wheat [31][36][37]. When hexose concentration is high, it likely reflects the action of sucrose-cleaving enzymes during sampling (invertase, sucrose synthase) and/or the contribution of hexose from petiole tissues to the extract obtained by exudation [38][39]. Amino acids are the second most abundant metabolites in phloem sap, representing a small percentage of the total sap concentration with substantial variations between species, from about 5% to 15% [15][31][40], up to 360 mM in maize [28]. The prevalent amino acids are glutamate (Glu), glutamine (Gln), aspartate (Asp) and asparagine (Asn) [17][20][23][32][36][41][42][43][44][45]. However, in other species, other amino acids have been found to be more abundant: proline (Pro), alanine (Ala) or glycine (Gly) in Arabidopsis, Citrus species or maize [15][24][27][28][40]. Interestingly, amino acid phloem sap composition changes with the photoperiod. Under long days, Glu, Asp, and Ser decrease while Gln and Asn increase compared to short days, in Arabidopsis [46]. Organic acids are also present in phloem sap, at rather small concentrations, depending on the species. In Arabidopsis, total organic acid concentration is less than 0.5 mM [31], i.e., about 5% of the total metabolites. The same amount (5%) has been found in maize [28] and Eucalyptus (5 mM, [23]) but higher content has been found in others, e.g., castor bean (30 to 47 mM [20]) and lemon tree (44 to 232 mM, [24]). Most represented organic acids are from the tricarboxylic acid pathway: malate, citrate and aconitate [23][28][32][35]. Quinate has also been found in Prunus [34] and Citrus [24] species. In squash (Cucurbita maxima), lactate is as concentrated as malate [32], and propanoate and maleate are as concentrated as malate in Arabidopsis [40]. Free fatty acids are often overlooked in phloem sap composition. However, they may be present at a higher content than organic acids in most species tested so far. For example, fatty acids and their derivatives reach 13% of total metabolites in Arabidopsis [40]. Their concentration has been found to be up to 5 mM in Citrus species [24][27]. Palmitate and oleate appear to be prevalent fatty acids, along with stearate (Citrus, Arabidopsis) [27][40][47] and specific oxylipins, and phospholipids were detected only when the EDTA method was used (in Arabidopsis) [48]. It has been suggested that sap collection methods can change fatty acid composition or perhaps explain their presence in sap samples.3. Metabolic Pathways Reflected by Phloem Sap Metabolome
It is worth noting that the diversity of metabolites found in phloem sap (and summarized in Section 3 above) reflects different metabolic pathways such as sugar metabolism, nitrogen and sulfur assimilation and amino acid synthesis, the tricarboxylic acid pathway, arginine metabolism and polyamine synthesis. There is presently some uncertainty as to whether some of these pathways take place in phloem cells themselves (CC and SE) or only reflect source cell metabolism. It is well-accepted that major amino acids and sugars come from source cell N assimilation and photosynthesis, respectively. Specific amino acid synthesis may occur in phloem cells themselves such as asparagine since a phloem isoform of asparagine synthetase has been found in Arabidopsis [49][50]. Pioneering studies have shown that several enzymatic activities are absent from phloem sap in particular those involved in sugar cleavage and utilization [51]. This is consistent with the fact that phloem sugar transport should not compete with sugar catabolism. Accordingly, it is generally believed that SE mitochondria are small and not numerous probably reflecting limited catabolic activity [52]. GC-MS-based metabolomics analysis of phloem sap in Citrus cultivars has shown that many metabolite contents change along with the total sap osmolarity [24]. This indicates that the increase in total sap concentration is not simply associated with a general increase in loading capability of source leaves but is also associated with modifications in metabolic pathways. This is readily visible via combined univariate-multivariate statistics, with total sap concentration as a response variable (Figure 12). As phloem sap concentration changes, sucrose covaries with organic acids including malate (cluster 2, Figure 12a, red arrow). Even so, the amino acids hexoses and inositol are more correlated to the total concentration than sucrose (Figure 12b). Amongst amino acids, arginine, aspartate and asparagine are good markers of a high phloem sap concentration while cysteine appears to be a marker of low phloem sap concentration. Metabolites that increase concurrently with total phloem sap concentration include pyruvate and glycolate (cluster 4, Figure 12a, blue arrow). Such changes are reminiscent of the aspartate ‘cycle’ (illustrated in Figure 12c, in red) which connects organic acid metabolism (malate) to aspartate via arginine biosynthesis.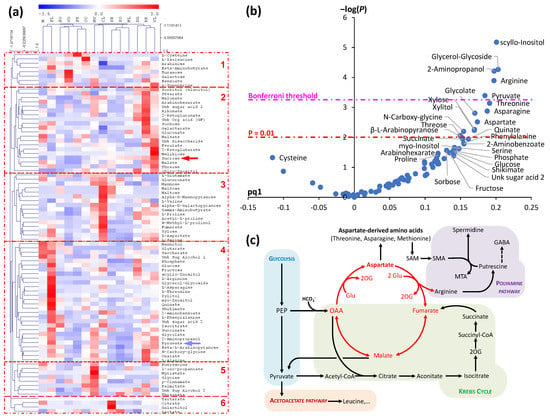
4. Phloem Sap Metabolites and Plant Resistance to Environmental Cues
Amino acid composition in phloem sap appears to respond to environmental factors such as nutrient availability. When supplied with high levels of nitrogen (N), Glu content increase in Arabidopsis phloem sap and conversely, Pro, Gln and γ-aminobutyrate (4-aminobutyrate; GABA) increase under low N availability [31]. Total amino acid concentration also changes with N supply as has been shown in canola [53]. Other conditions such as low phosphorus (P) availability and water deficit also affect amino acid composition [31][54]. Polyol content in phloem sap (such as sorbitol and erythritol) have also been found to impact drought and cold tolerance [24][28]. Recently, metabolomics analyses of cucumber plants subjected to phosphorus deficiency have shown important changes in phloem sap metabolome, not only in carbohydrates (galactitol, fructose) but also in organic acids (e.g., oxalate, citrate, fatty acids) along with nitrogenous compounds (e.g., ethanolamine, 4-aminobutyrate and pyroglutamate) [33]. Metabolomics analyses of phloem sap exudates during root waterlogging have found a change in the sugar to organic acid ratio, suggesting (i) a crucial role of the balance between loading in shoots and unloading in roots for sap composition and (ii) potentially, a negative feedback of metabolite accumulation in phloem sap onto shoot metabolism, such as sugar interconversions and respiration [55].
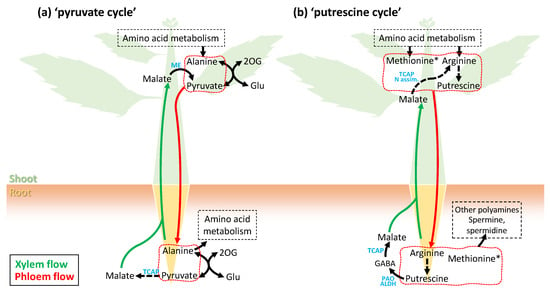
5. Phloem Sap Metabolome: Unforeseen Whole-Plant Metabolic Cycles?
References
- Dinant, S.; Lemoine, R. The phloem pathway: New issues and old debates. Comptes Rendus Biol. 2010, 333, 307–319.
- Evert, R.F. Phloem Structure and Histochemistry. Annu. Rev. Plant Physiol. 1977, 28, 199–222.
- Cronshaw, J. Phloem Structure and Function. Annu. Rev. Plant Physiol. 1981, 32, 465–484.
- Golecki, B.; Schulz, A.; Thompson, G.A. Translocation of Structural P Proteins in the Phloem. Plant Cell 1999, 11, 127–140.
- Ernst, A.M.; Jekat, S.B.; Zielonka, S.; Muller, B.; Neumann, U.; Ruping, B.; Twyman, R.M.; Krzyzanek, V.; Prufer, D.; Noll, G.A. Sieve element occlusion (SEO) genes encode structural phloem proteins involved in wound sealing of the phloem. Proc. Natl. Acad. Sci. USA 2012, 109, E1980–E1989.
- Sellier, D.; Harrington, J.J. Phloem transport in trees: A generic surface model. Ecol. Model. 2014, 290, 102–109.
- Holbrook, N.M.; Knoblauch, M. Editorial overview: Physiology and metabolism: Phloem: A supracellular highway for the transport of sugars, signals, and pathogens. Curr. Opin. Plant Biol. 2018, 43, iii–vii.
- Weiler, E.W. Determination of phytohormones in phloem exudate from tree species by radioimmunoassay. Planta 1981, 152, 168–170.
- Simpson, R.J. Nitrogen Redistribution during Grain Growth in Wheat (Triticum aestivum L.) 1: IV. Development of a Quantitative Model of the Translocation of Nitrogen to the Grain. Plant Physiol. 1983, 71, 7–14.
- Rennenberg, H. Analysis of uptake and allocation of nitrogen and sulphur compounds by trees in the field. J. Exp. Bot. 1996, 47, 1491–1498.
- Anderson, J.W. Sulphur Distribution and Redistribution in Vegetative and Generative Plants. In Sulphur in Plants; Springer: Dordrecht, The Netherlands, 2003; pp. 113–134.
- Youssefi, F.; Brown, P.; Weinbaum, S. Relationship between tree nitrogen status, xylem and phloem sap amino acid concentrations, and apparent soil nitrogen uptake by almond trees (Prunus dulcis). J. Hortic. Sci. Biotechnol. 2015, 75, 62–68.
- Pate, J.S.; Jeschke, W.D. Mineral uptake and transport in xylem and phloem of the proteaceous tree, Banksia prionotes. Plant Soil 1993, 155, 273–276.
- Lohaus, G. Review primary and secondary metabolites in phloem sap collected with aphid stylectomy. J. Plant Physiol. 2022, 271, 153645.
- Ohshima, T.; Hayashi, H.; Chino, M. Collection and Chemical Composition of Pure Phloem Sap from Zea mays L. Plant Cell Physiol. 1990, 31, 735–737.
- Lohaus, G.; Moellers, C. Phloem transport of amino acids in two Brassica napus L. genotypes and one B. carinata genotype in relation to their seed protein content. Planta 2000, 211, 833–840.
- Hayashi, H.; Chino, M. Chemical Composition of Phloem Sap from the Uppermost Internode of the Rice Plant. Plant Cell Physiol. 1990, 31, 247–251.
- Winter, H.; Lohaus, G.; Heldt, H.W. Phloem Transport of Amino Acids in Relation to their Cytosolic Levels in Barley Leaves 1. Plant Physiol. 1992, 99, 996–1004.
- Hayashi, H.; Chino, M. Collection of Pure Phloem Sap from Wheat and its Chemical Composition. Plant Cell Physiol. 1986, 27, 1387–1393.
- Hall, S.M.; Baker, D.A. The chemical composition of Ricinus phloem exudate. Planta 1972, 106, 131–140.
- Oner-Sieben, S.; Lohaus, G. Apoplastic and symplastic phloem loading in Quercus robur and Fraxinus excelsior. J. Exp. Bot. 2014, 65, 1905–1916.
- Fink, D.; Dobbelstein, E.; Barbian, A.; Lohaus, G. Ratio of sugar concentrations in the phloem sap and the cytosol of mesophyll cells in different tree species as an indicator of the phloem loading mechanism. Planta 2018, 248, 661–673.
- Pate, J.; Shedley, E.; Arthur, D.; Adams, M. Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus. Oecologia 1998, 117, 312–322.
- Killiny, N. Metabolite signature of the phloem sap of fourteen citrus varieties with different degrees of tolerance to Candidatus liberibacter asiaticus. Physiol. Mol. Plant Pathol. 2017, 97, 20–29.
- Heineke, D.; Sonnewald, U.; Büssis, D.; Günter, G.; Leidreiter, K.; Wilke, I.; Raschke, K.; Willmitzer, L.; Heldt, H.W. Apoplastic Expression of Yeast-Derived Invertase in Potato: Effects on Photosynthesis, Leaf Solute Composition, Water Relations, and Tuber Composition. Plant Physiol. 1992, 100, 301–308.
- Winzer, T.; Lohaus, G.; Heldt, H.-W. Influence of phloem transport, N-fertilization and ion accumulation on sucrose storage in the taproots of fodder beet and sugar beet. J. Exp. Bot. 1996, 47, 863–870.
- Hijaz, F.; Killiny, N. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS ONE 2014, 9, e101830.
- Yesbergenova-Cuny, Z.; Dinant, S.; Martin-Magniette, M.-L.; Quilleré, I.; Armengaud, P.; Monfalet, P.; Lea, P.J.; Hirel, B. Genetic variability of the phloem sap metabolite content of maize (Zea mays L.) during the kernel-filling period. Plant Sci. 2016, 252, 347–357.
- Stallmann, J.; Schweiger, R. Effects of Arbuscular Mycorrhiza on Primary Metabolites in Phloem Exudates of Plantago major and Poa annua and on a Generalist Aphid. Int. J. Mol. Sci. 2021, 22, 13086.
- Tedesco, S.; Erban, A.; Gupta, S.; Kopka, J.; Fevereiro, P.; Kragler, F.; Pina, A. The Impact of Metabolic Scion-Rootstock Interactions in Different Grapevine Tissues and Phloem Exudates. Metabolites 2021, 11, 349.
- Chardon, F.; De Marco, F.; Marmagne, A.; Le Hir, R.; Vilaine, F.; Bellini, C.; Dinant, S. Natural variation in the long-distance transport of nutrients and photoassimilates in response to N availability. J. Plant Physiol. 2022, 273, 153707.
- Fiehn, O. Metabolic networks of Cucurbita maxima phloem. Phytochemistry 2003, 62, 875–886.
- Sun, J.; Li, Q.; Xu, H.; Zhang, W. Analysis of Metabolomic Changes in Xylem and Phloem Sap of Cucumber under Phosphorus Stresses. Metabolites 2022, 12, 361.
- Gallinger, J.; Gross, J. Phloem Metabolites of Prunus sp. Rather than Infection with Candidatus phytoplasma prunorum Influence Feeding Behavior of Cacopsylla pruni Nymphs. J. Chem. Ecol. 2020, 46, 756–770.
- Peuke, A. Correlations in concentrations, xylem and phloem flows, and partitioning of elements and ions in intact plants. A summary and statistical re-evaluation of modelling experiments in Ricinus communis. J. Exp. Bot. 2010, 61, 635–655.
- Palmer, L.J.; Dias, D.A.; Boughton, B.; Roessner, U.; Graham, R.D.; Stangoulis, J.R. Metabolite profiling of wheat (Triticum aestivum L.) phloem exudate. Plant Methods 2014, 10, 27.
- Xie, H.; Shi, F.; Li, J.; Yu, M.; Yang, X.; Li, Y.; Fan, J. The Reciprocal Effect of Elevated CO2 and Drought on Wheat-Aphid Interaction System. Front. Plant Sci. 2022, 13, 853220.
- Turgeon, R.; Wolf, S. Phloem transport: Cellular pathways and molecular trafficking. Annu. Rev. Plant Biol. 2009, 60, 207–221.
- Liu, D.D.; Chao, W.M.; Turgeon, R. Transport of sucrose, not hexose, in the phloem. J. Exp. Bot. 2012, 63, 4315–4320.
- Guelette, B.S.; Benning, U.F.; Hoffmann-Benning, S. Identification of lipids and lipid-binding proteins in phloem exudates from Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 3603–3616.
- Valle, E.M.; Boggio, S.B.; Heldt, H.W. Free Amino Acid Composition of Phloem Sap and Growing Fruit of Lycopersicon esculentum. Plant Cell Physiol. 1998, 39, 458–461.
- Schneider, S.; GessLer, A.; Weber, P.; Sengbusch, D.; Hanemann, U.; Rennenberg, H. Soluble N compounds in trees exposed to high loads of N: A comparison of spruce (Picea abies) and beech (Fagus sylvatica) grown under field conditions. New Phytol. 1996, 134, 103–114.
- Hocking, P.J. The Composition of Phloem Exudate and Xylem Sap from Tree Tobacco (Nicotiana glauca Grah.). Ann. Bot. 1980, 45, 633–643.
- Lohaus, G.; Büker, M.; Hußmann, M.; Soave, C.; Heldt, H.-W. Transport of amino acids with special emphasis on the synthesis and transport of asparagine in the Illinois Low Protein and Illinois High Protein strains of maize. Planta 1998, 205, 181–188.
- Sandström, J.; Pettersson, J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J. Insect Physiol. 1994, 40, 947–955.
- Corbesier, L.; Havelange, A.; Lejeune, P.; Bernier, G.; Périlleux, C. N content of phloem and xylem exudates during the transition to flowering in Sinapis alba and Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 367–375.
- Valim, M.F.; Killiny, N. Occurrence of free fatty acids in the phloem sap of different citrus varieties. Plant Signal. Behav. 2017, 12, e1327497.
- Hoffmann-Benning, S. Collection and Analysis of Phloem Lipids. Methods Mol. Biol. 2021, 2295, 351–361.
- Gaufichon, L.; Reisdorf-Cren, M.; Rothstein, S.; Chardon, F.; Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010, 179, 141–153.
- Gaufichon, L.; Masclaux-Daubresse, C.; Tcherkez, G.; Reisdorf-Cren, M.; Sakakibara, Y.; Hase, T.; Clement, G.; Avice, J.C.; Grandjean, O.; Marmagne, A.; et al. Arabidopsis thaliana ASN2 encoding asparagine synthetase is involved in the control of nitrogen assimilation and export during vegetative growth. Plant Cell Environ. 2013, 36, 328–342.
- Kennecke, M.; Ziegler, H.; de Fekete, M.A. Enzyme activities in the sieve tube sap of Robinia pseudoacacia L. and of other tree species. Planta 1971, 98, 330–356.
- Knoblauch, M. Sieve Tubes in Action. Plant Cell 1998, 10, 35–50.
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 2005, 221, 328–338.
- Girousse, C.; Bournoville, R.; Bonnemain, J.L. Water Deficit-Induced Changes in Concentrations in Proline and Some Other Amino Acids in the Phloem Sap of Alfalfa. Plant Physiol. 1996, 111, 109–113.
- Lothier, J.; Diab, H.; Cukier, C.; Limami, A.M.; Tcherkez, G. Metabolic Responses to Waterlogging Differ between Roots and Shoots and Reflect Phloem Transport Alteration in Medicago truncatula. Plants 2020, 9, 1373.
- van Beusichem, M.L.; Nelemans, O.A.; Hinnen, M.G.J. Nitrogen: Nitrogen cycling in plant species differing in shoot/root reduction of nitrate. J. Plant Nutr. 1987, 10, 1723–1731.
- Marschner, P.; Crowley, D.E.; Higashi, R.M. Root exudation and physiological status of a root-colonizing fluorescent pseudomonad in mycorrhizal and non-mycorrhizal pepper (Capsicum annuum L.). Plant Soil 1997, 189, 11–20.
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32.
- Trumbore, S.E.; Angert, A.; Kunert, N.; Muhr, J.; Chambers, J.Q. What’s the flux? Unraveling how CO2 fluxes from trees reflect underlying physiological processes. New Phytol. 2013, 197, 353–355.
