Possible new ways for the development of novel classes of antibiotics are discussed here, for which there is no pre-existing resistance in human bacterial pathogens. By utilizing research and technology such as nanotechnology and computational methods (such as in silico and Fragment-based drug design (FBDD)), there has been an improvement in antimicrobial actions and selectivity with target sites. Moreover, there are antibiotic alternatives, such as antimicrobial peptides, essential oils, anti-Quorum sensing agents, darobactins, vitamin B6, bacteriophages, odilorhabdins, 18β-glycyrrhetinic acid, and cannabinoids. Additionally, drug repurposing (such as with ticagrelor, mitomycin C, auranofin, pentamidine, and zidovudine) and synthesis of novel antibacterial agents (including lactones, piperidinol, sugar-based bactericides, isoxazole, carbazole, pyrimidine, and pyrazole derivatives) represent novel approaches to treating infectious diseases. Nonetheless, prodrugs (e.g., siderophores) have shown to be an excellent platform to design a new generation of antimicrobial agents with better efficacy against multidrug-resistant bacteria.
- antibiotic
- resistance
- antimicrobial agents
1. Introduction
2. Antibiotic Classification
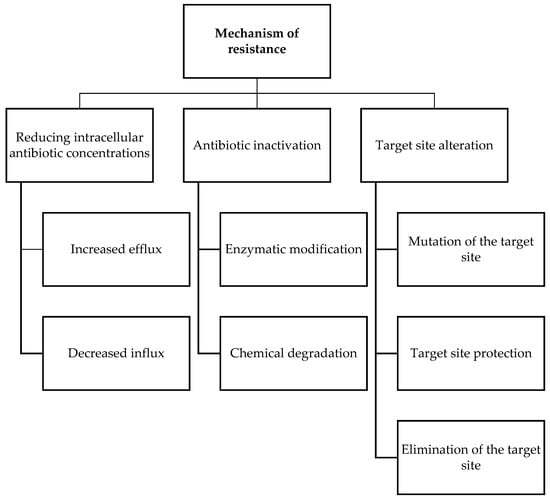
New antibiotic use methods should be implemented on a local and global level to combat resistance, and the development of novel treatments requires a thorough understanding of how antibiotics function. Table 1 summarizes how antibiotics produce their effects through a variety of mechanism of action. Antibiotic-mediated cell death is a complicated process that begins with a physical interaction between the medication and its particular target in bacteria, altering the bacterium’s biochemical, molecular, or ultrastructural levels. The development of DNA double-stranded DNA breaks, the halting of DNA-dependent RNA synthesis, cell envelop damage, protein mistranslation, and stress induction are only a few of the methods through which antibiotics can cause cell death [12]. Antimicrobial agents are divided into two categories on the basis of how they affect bacteria in a test tube: (1) bactericidal (kill bacteria) antibiotics such as β-lactams, glycopeptides, lipopeptides, rifamycins, aminoglycosides, and fluoroquinolones, and (2) bacteriostatic (prevent bacterial growth) antibiotics such as sulfonamides–trimethoprim and macrolides. Bacteriostatic substances can also be described as having a minimum bactericidal concentration (MBC) to minimum inhibitory concentration (MIC) ratio higher than four, and bactericidal substances when the MBC to MIC ratio is lower than or equal to four [13].|
Antibiotics Family |
Mechanism of Action |
Antibiotics |
|---|---|---|
|
β-lactam |
Binds to the serine active site of penicillin-binding proteins (PBPs) or the allosteric site in PBP2a to inhibit bacterial cell wall peptidoglycan transpeptidation [14][15][14,15]. |
Penicillins Cephalosporins Carbapenems Monocyclic β-lactams β-lactamase inhibitors (e.g., clavulanic acid) (Figure 1) |
|
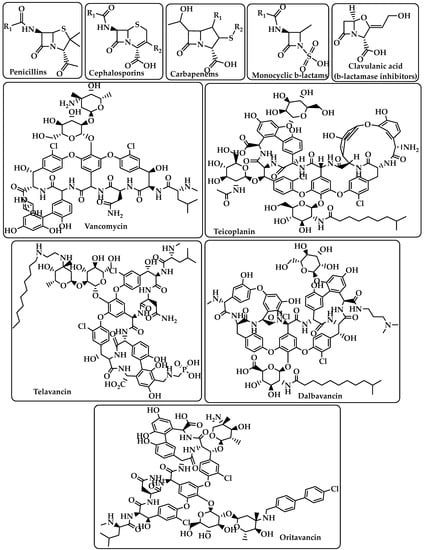
Glycopeptides |
Interacts with the membrane-bound lipid II precursor of peptidogly and can prevent peptidoglycan from being incorporated into an essential structural cell wall component [16]. |
Vancomycin Teicoplanin Telavancin Dalbavancin Oritavancin (Figure 1) |
|
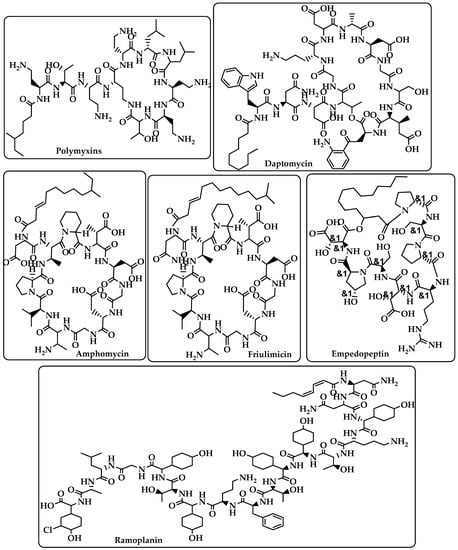
Lipopeptide |
Carries out their action by causing Gram-positive bacteria’s cell membrane integrity to be compromised, which results in cell death [17][18][17,18]. |
Polymyxins Daptomycin Amphomycin Friulimicin Ramoplanin Empedopeptin (Figure 2) |
|
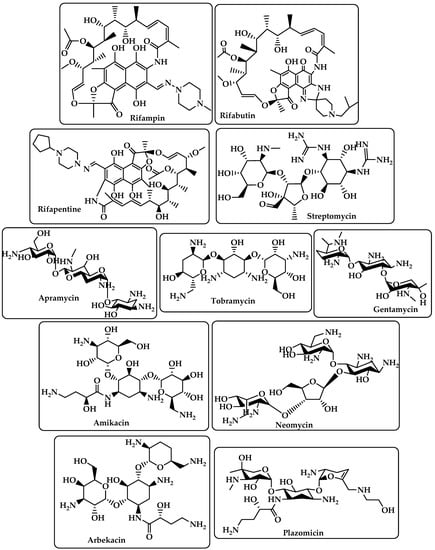
Rifamycins |
RNA polymerase (RNAP) inhibitors are used to treat tuberculosis (TB) [19]. |
Rifampin Rifabutin Rifapentine (Figure 3) |
|
Aminoglycoside |
By attaching to the 30S ribosome’s A-site on the 16S ribosomal RNA, they inhibit protein synthesis [20]. |
Streptomycin Apramycin Tobramycin Gentamcin Amikacin Neomycin Arbekacin Plazomicin (Figure 3) |
|
Fluoroquinolones |
Target DNA gyrase, topoisomerase IV, and topoisomerase type II to prevent bacteria from synthesizing DNA [21]. |
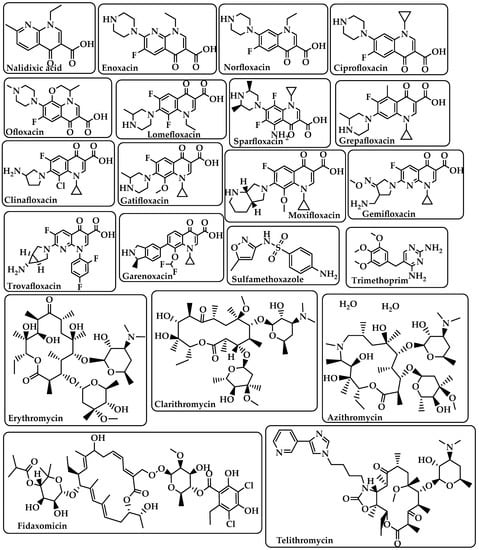
Nalidixic acid Enoxacin Norfloxacin Ciprofloxacin Ofloxacin Lomefloxacin Sparfloxacin Grepafloxacin Clinafloxacin Gatifloxacin Moxifloxacin Gemifloxacin Trovafloxacin Garenoxacin (Figure 4) |
|
Sulfonamides–Trimethoprim |
Sulfonamides interfere with the activity of the dihydropteroate synthase enzyme by competing with p-aminobenzoic acid (PABA) in the process of dihydrofolate production.The dihydrofolate reductase enzyme is inhibited by trimethoprim because it competes directly with it [22]. |
Sulfamethoxazole Trimethoprim (Figure 4) |
|
Macrolides |
Target the nascent peptide exit tunnel (NPET) of the bacterial 50S ribosomal subunit, which is responsible for the release of newly synthesized protein from the ribosome, ultimately preventing protein synthesis [23][24][23,24]. |
Erythromycin Clarithromycin Azithromycin Fidaxomicin Telithromycin (Figure 4) |
|
Tetracyclines |
Bind to the small subunit’s decoding site and prevent bacterial protein synthesis [25][26][25,26]. |
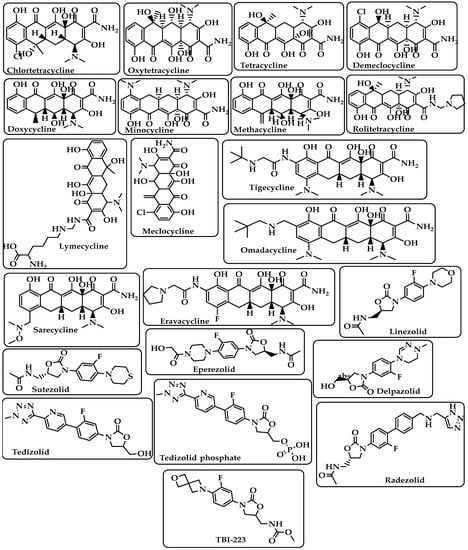
Chlortetracycline Oxytetracycline Tetracycline Demeclocycline Doxycycline Minocycline Lymecycline Meclocycline Methacycline RolitetracyclineTigecycline Omadacycline Sarecycline Eravacycline (Figure 5) |
|
Oxazolidinones |
Block the translation sequence by interacting with the 50S subunit (A-site pocket) at the peptidyl transferase center (PTC) to inhibit protein synthesis [27]. |
Linezolid Sutezolid Eperezolid Delpazolid Tedizolid Tedizolid phosphate Radezolid TBI-223 (Figure 5) |
|
Streptogramins |
Inhibit protein synthesis during the elongation step by attaching to bacterial ribosomes [28]. The antibiotic has two unique structural groups (A and B) that cooperate to increase the affinity of group B in the nearby nascent peptide exit tunnel (NPET) when group A binds to the peptidyl transferase center (PTC) [29]. |
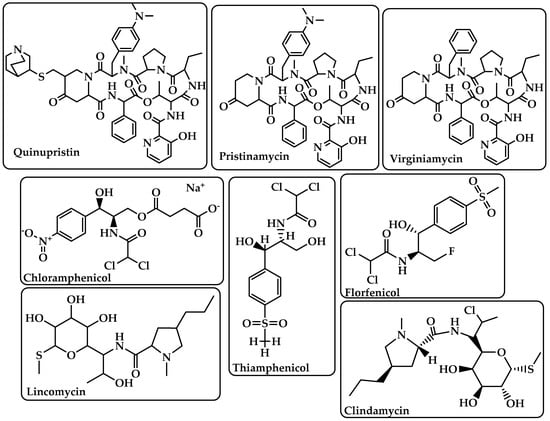
Quinupristin Pristinamycin Virginiamycin (Figure 6) |
|
Phenicoles |
Inhibit protein synthesis by binding to the 50S ribosomal subunit [30]. |
Chloramphenicol Thiamphenicol Florfenicol (Figure 6) |
|
Lincosamides |
Activate amino acid monomers by aminoacyl-tRNA, chain initiation, elongation, and termination of the formed polypeptides on the ribosome, which disrupts bacterial growth and death. These are only a few of the many processes that can be affected to prevent microbial protein synthesis [31]. |
Lincomycin Clindamycin (Figure 6) |
3. Antimicrobial Resistance