The Golgi associated retrograde protein complex (GARP) is an evolutionarily conserved component of Golgi membrane trafficking machinery that belongs to the Complexes Associated with Tethering Containing Helical Rods (CATCHR) family.
- Golgi
- vesicle
- glycosylation
- GARP complex
- VPS51
- VPS52
- VPS53
- VPS54
- SNARE
1. Composition and Structure of the Golgi ARPssociated Retrograde Protein Complex
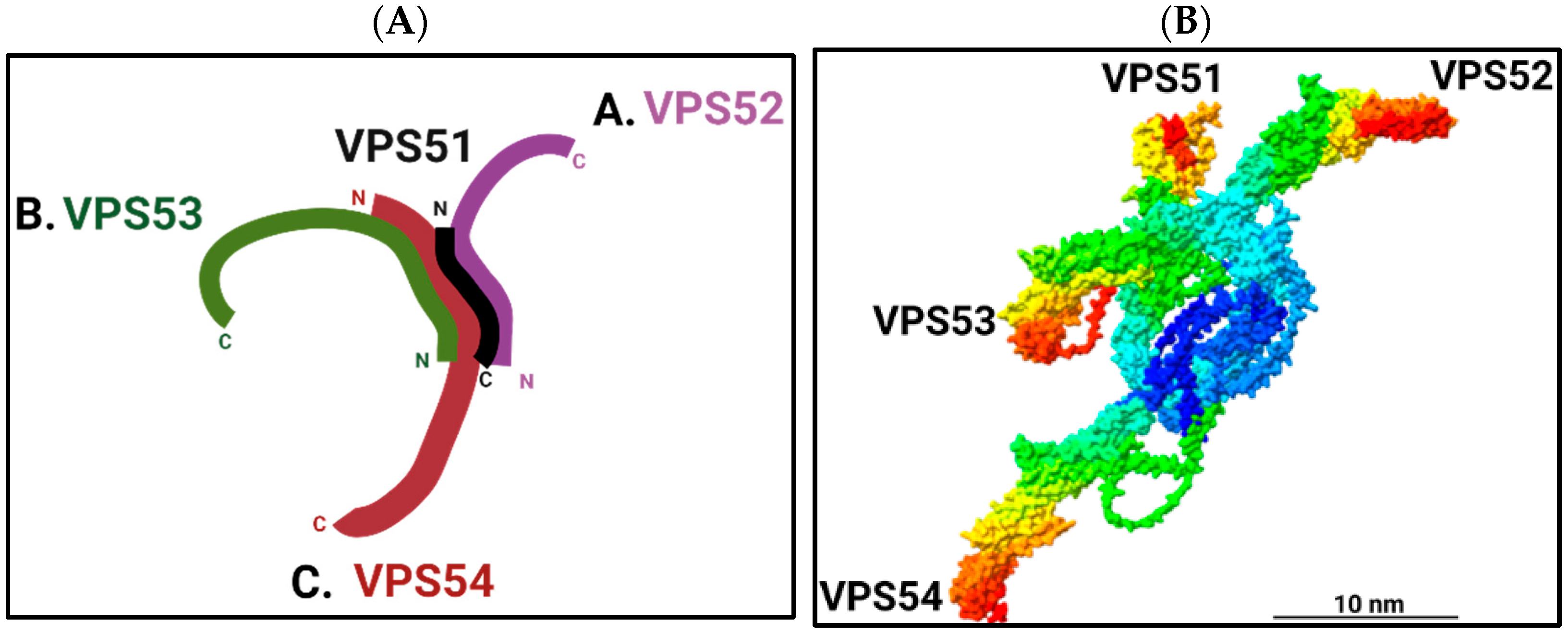
2. Functions of the Golgi ARPssociated Retrograde Protein Complex
2.1. GARP as a Molecular Tether for the Endosomal-Derived Vesicles
2.1. Golgi Associated Retrograde Protein as a Molecular Tether for the Endosomal-Derived Vesicles
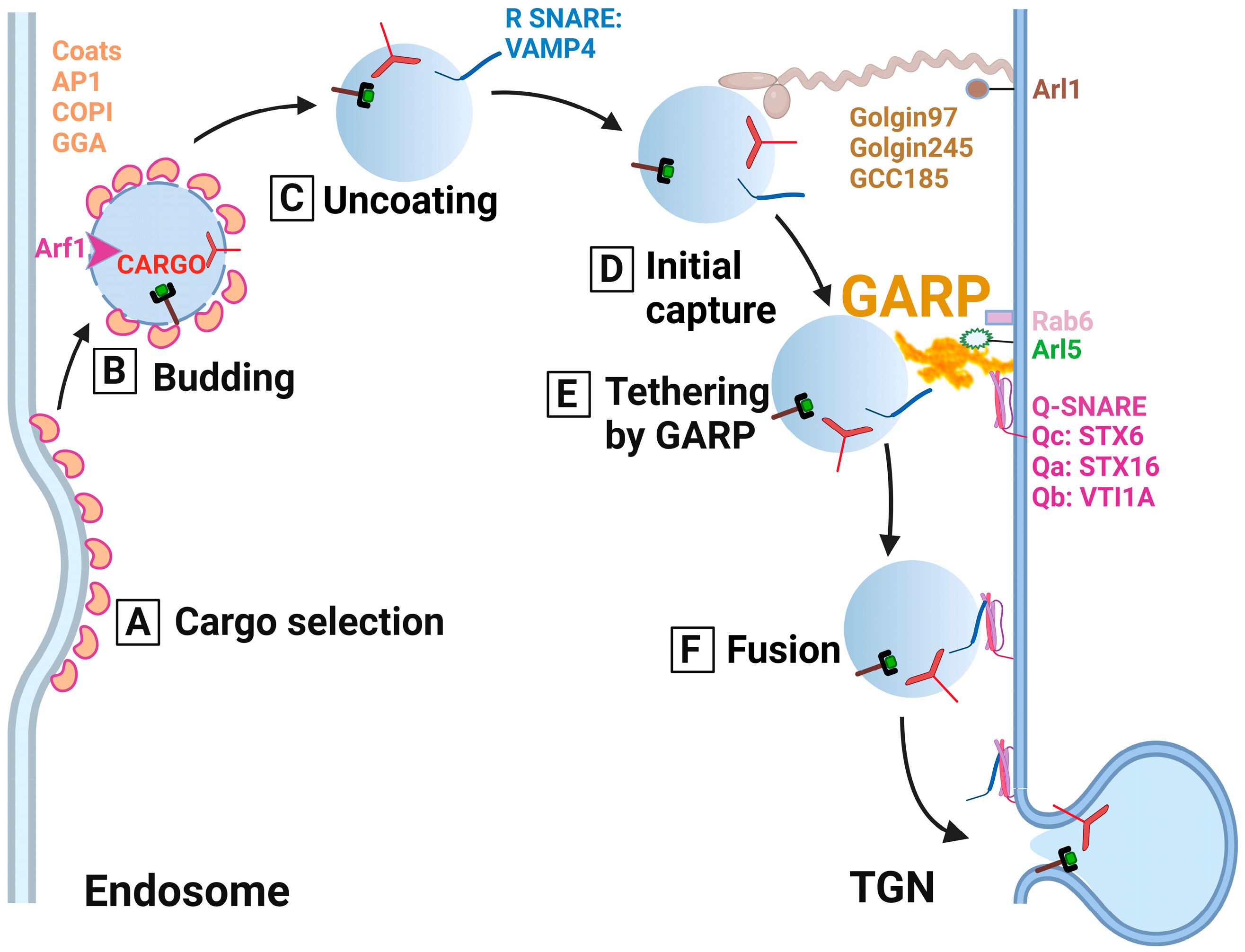
2.2. GARP as a Regulator of SNARE Complexes
2.2. Golgi Associated Retrograde Protein as a Regulator of SNARE Complexes
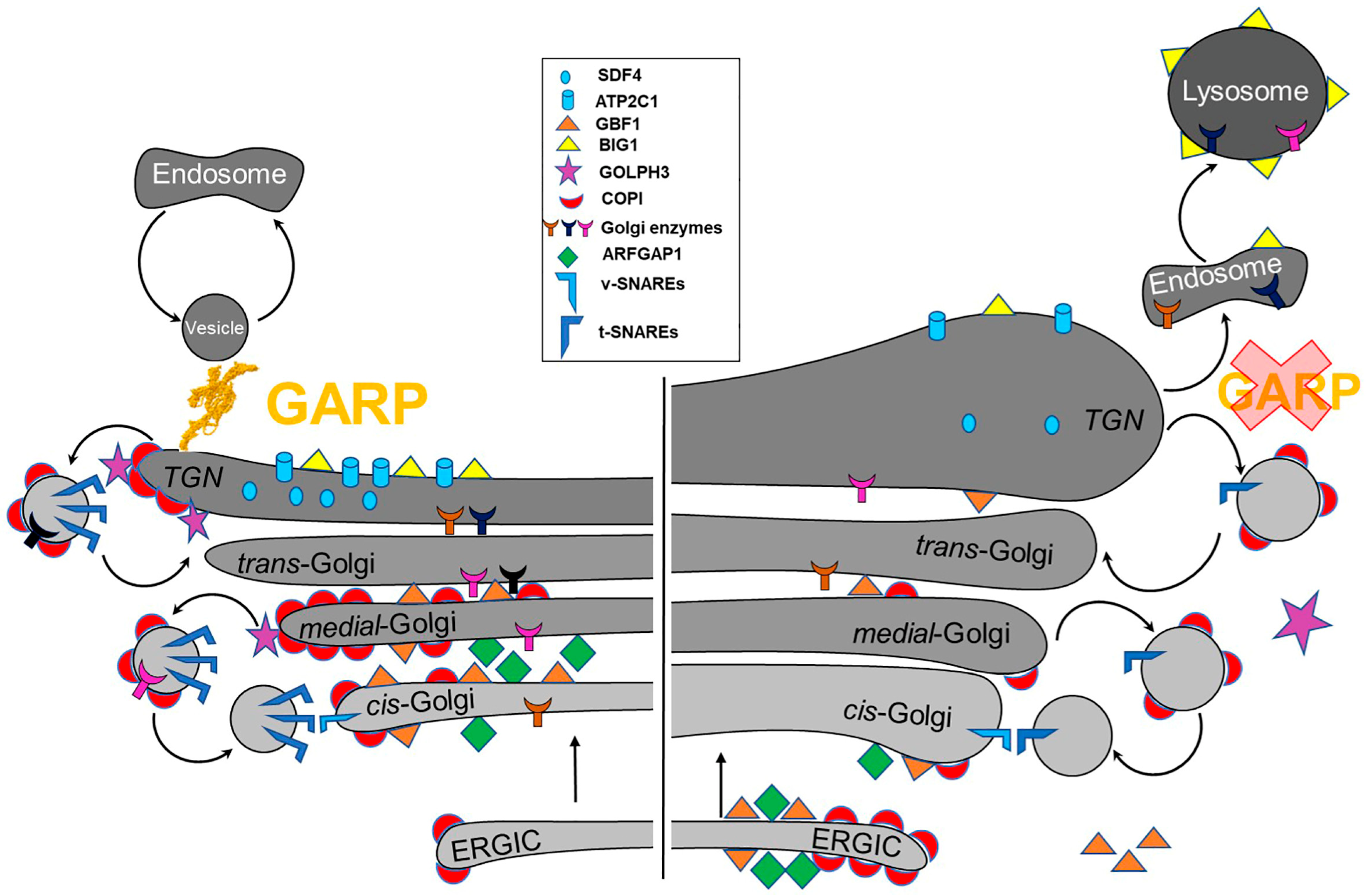
2.3. Role of the GARP Complex in the Maintenance of Golgi Glycosylation Machinery
2.3. Role of the Golgi Associated Retrograde Protein Complex in the Maintenance of Golgi Glycosylation Machinery
2.4. Role of the GARP Complex in Normal Golgi Physiology
2.4. Role of the Golgi Associated Retrograde Protein Complex in Normal Golgi Physiology
2.5. GARP and Lipid Homeostasis
2.5. Golgi Associated Retrograde Protein and Lipid Homeostasis
2.6. Role of GARP Complex in the Secretory Pathway
2.6. Role of Golgi Associated Retrograde Protein Complex in the Secretory Pathway
2.7. Hijacking of GARP by Intracellular Pathogens
2.7. Hijacking of Golgi Associated Retrograde Protein by Intracellular Pathogens
2.8. Future perspectives in GARP
Golgi Associated Retrograde Protein
studies
Despite being discovered over two decades ago, our understanding of the GARP complex remains limited. To fully comprehend its function, it is crucial to investigate the complex's dynamic nature and elucidate its overall and subunit architecture, especially for the mammalian GARP complex in both free and membrane-bound states. Understanding how GARP switches from a "close" to an "open" conformation to tether incoming transport intermediates is critical.
The shared VPS51/52/53 trimer between the GARP and EARP complexes raises questions about how differences in one subunit between these complexes affect their localization and function. It is unclear whether the shared trimer is dynamically switching between GARP and EARP conformations or if the two complexes are stable and do not exchange subunits. Additionally, identifying the factors that make GARP unique from EARP, such as SNAREs, coiled-coil tethers, and small GTPases, is necessary.
Although defects in GARP subunits disrupt endosome-TGN trafficking, studies have shown that mutant phenotypes extend beyond this, affecting glycosylation, intra-Golgi transport, and secretion. Understanding the immediate and secondary impacts of GARP dysfunction is crucial.
Direct evidence of GARP's ability to capture specific transport vesicles is still lacking. It is unclear whether GARP captures vesicles independently or in conjunction with coiled-coil tethers and whether it captures only one type of transport vesicle. Identifying the specific protein and lipid cargo that relies on the GARP complex is critical. Isolating and further characterizing GARP-dependent membrane carriers and exploring the interplay between GARP and other players in TGN vesicle tethering, docking, and fusion machinery is essential.
While it is suggested that GARP promotes the assembly of TGN SNAREs, the exact mode of interaction between GARP and SNAREs is unclear. Further research is needed to elucidate the precise mechanisms by which GARP functions in the cell, such as whether it only interacts with the STX16 SNARE complex or binds and regulates the STX16-interacting SM protein VPS45.
The dysfunction of the GARP complex can result in pathogenesis in humans, mice, and plants. VPS54 null causes embryonic lethality in mice, raising questions about the specific functions of GARP in different cell types and how its dysfunction can lead to such severe outcomes. Furthermore, it is unclear why GARP deficiency primarily affects neuronal function and how the complex is involved in various signaling pathways associated with cancer. To gain a more comprehensive understanding of GARP functions and dysfunctions, it is imperative to study the complex in a range of human tissues and other species beyond yeast and mammalian cell models.
References
- Brocker, C.; Engelbrecht-Vandre, S.; Ungermann, C. Multisubunit tethering complexes and their role in membrane fusion. Curr. Biol. 2010, 20, R943–R952.
- Bonifacino, J.S.; Hierro, A. Transport according to GARP: Receiving retrograde cargo at the trans-Golgi network. Trends Cell Biol. 2011, 21, 159–167.
- Conibear, E.; Stevens, T.H. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol. Biol. Cell 2000, 11, 305–323.
- Siniossoglou, S.; Pelham, H.R.B. Vps51p links the VFT complex to the SNARE Tlg1p. J. Biol. Chem. 2002, 277, 48318–48324.
- Pérez-Victoria, F.J.; Schindler, C.; Magadán, J.G.; Mardones, G.A.; Delevoye, C.; Romao, M.; Bonifacino, J.S. Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex. Mol. Biol. Cell 2010, 21, 3386–3395.
- Chou, H.-T.; Dukovski, D.; Chambers, M.G.; Reinisch, K.M.; Walz, T. CATCHR, HOPS and CORVET tethering complexes share a similar architecture. Nat. Struct. Mol. Biol. 2016, 23, 761–763.
- Schindler, C.; Chen, Y.; Pu, J.; Guo, X.; Bonifacino, J.S. EARP is a multisubunit tethering complex involved in endocytic recycling. Nat. Cell Biol. 2015, 17, 639–650.
- Topalidou, I.; Cattin-Ortolá, J.; Pappas, A.L.; Cooper, K.; Merrihew, G.E.; MacCoss, M.J.; Ailion, M. The EARP complex and its interactor EIPR-1 are required for cargo sorting to dense-core vesicles. PLoS Genet. 2016, 12, e1006074.
- Vasan, N.; Hutagalung, A.; Novick, P.; Reinisch, K.M. Structure of a C-terminal fragment of its Vps53 subunit suggests similarity of Golgi-associated retrograde protein (GARP) complex to a family of tethering complexes. Proc. Natl. Acad. Sci. USA 2010, 107, 14176–14181.
- Pei, J.; Ma, C.; Rizo, J.; Grishin, N.V. Remote homology between Munc13 MUN domain and vesicle tethering complexes. J. Mol. Biol. 2009, 391, 509–517.
- Augustin, I.; Rosenmund, C.; Südhof, T.C.; Brose, N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 1999, 400, 457–461.
- Basu, J.; Shen, N.; Dulubova, I.; Lu, J.; Guan, R.; Guryev, O.; Grishin, N.V.; Rosenmund, C.; Rizo, J. A minimal domain responsible for Munc13 activity. Nat. Struct. Mol. Biol. 2005, 12, 1017–1018.
- Pérez-Victoria, F.J.; Abascal-Palacios, G.; Tascón, I.; Kajava, A.; Magadán, J.G.; Pioro, E.P.; Bonifacino, J.S.; Hierro, A. Structural basis for the wobbler mouse neurodegenerative disorder caused by mutation in the Vps54 subunit of the GARP complex. Proc. Natl. Acad. Sci. USA 2010, 107, 12860–12865.
- Whyte, J.R.; Munro, S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 2001, 1, 527–537.
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682.
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82.
- Conibear, E.; Cleck, J.N.; Stevens, T.H. Vps51p mediates the association of the GARP (Vps52/53/54) complex with the late Golgi t-SNARE Tlg1p. Mol. Biol. Cell 2003, 14, 1610–1623.
- Reggiori, F.; Wang, C.-W.; Stromhaug, P.E.; Shintani, T.; Klionsky, D.J. Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion. J. Biol. Chem. 2003, 278, 5009–5020.
- Fabrizio, J.; Hime, G.; Lemmon, S.; Bazinet, C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development 1998, 125, 1833–1843.
- Wilkinson, E.C.; Starke, E.L.; Barbee, S.A. Vps54 Regulates Lifespan and Locomotor Behavior in Adult Drosophila melanogaster. Front. Genet. 2021, 12, 762012.
- Schmitt-John, T.; Drepper, C.; Mußmann, A.; Hahn, P.; Kuhlmann, M.; Thiel, C.; Hafner, M.; Lengeling, A.; Heimann, P.; Jones, J.M.; et al. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat. Genet. 2005, 37, 1213–1215.
- Pérez-Victoria, F.J.; Mardones, G.A.; Bonifacino, J.S. Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol. Biol. Cell 2008, 19, 2350–2362.
- Pérez-Victoria, F.J.; Bonifacino, J.S. Dual roles of the mammalian GARP complex in tethering and SNARE complex assembly at the trans-golgi network. Mol. Cell. Biol. 2009, 29, 5251–5263.
- Khakurel, A.; Kudlyk, T.; Bonifacino, J.S.; Lupashin, V.V. The Golgi-associated retrograde protein (GARP) complex plays an essential role in the maintenance of the Golgi glycosylation machinery. Mol. Biol. Cell 2021, 32, 1594–1610.
- Conboy, M.J.; Cyert, M.S. Luv1p/Rki1p/Tcs3p/Vps54p, a yeast protein that localizes to the late Golgi and early endosome, is required for normal vacuolar morphology. Mol. Biol. Cell 2000, 11, 2429–2443.
- Shestakova, A.; Suvorova, E.; Pavliv, O.; Khaidakova, G.; Lupashin, V. Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J. Cell Biol. 2007, 179, 1179–1192.
- Peer, M.; Yuan, H.; Zhang, Y.; Korbula, K.; Novick, P.; Dong, G. Double NPY motifs at the N-terminus of the yeast t-SNARE Sso2 synergistically bind Sec3 to promote membrane fusion. eLife 2022, 11, e82041.
- Travis, S.M.; Damico, K.; Yu, I.-M.; McMahon, C.; Hamid, S.; Ramirez-Arellano, G.; Jeffrey, P.D.; Hughson, F.M. Structural basis for the binding of SNAREs to the multisubunit tethering complex Dsl1. J. Biol. Chem. 2020, 295, 10125–10135.
- Torng, T.; Wickner, W. Phosphatidylinositol and phosphatidylinositol-3-phosphate activate HOPS to catalyze SNARE assembly, allowing small headgroup lipids to support the terminal steps of membrane fusion. Mol. Biol. Cell 2021, 32, ar19.
- Hong, W.; Lev, S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 2014, 24, 35–43.
- Khakurel, A.; Kudlyk, T.; Pokrovskaya, I.; D’Souza, Z.; Lupashin, V.V. GARP dysfunction results in COPI displacement, depletion of Golgi v-SNAREs and calcium homeostasis proteins. Front. Cell Dev. Biol. 2022, 10, 1066504.
- Smith, R.D.; Lupashin, V.V. Role of the conserved oligomeric Golgi (COG) complex in protein glycosylation. Carbohydr. Res. 2008, 343, 2024–2031.
- Brasil, S.; Pascoal, C.; Francisco, R.; Marques-da-Silva, D.; Andreotti, G.; Videira, P.A.; Morava, E.; Jaeken, J.; Dos Reis Ferreira, V. CDG therapies: From bench to bedside. Int. J. Mol. Sci. 2018, 19, 1304.
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199.
- Gershlick, D.; Ishida, M.; Jones, J.R.; Bellomo, A.; Bonifacino, J.S.; Everman, D.B. A neurodevelopmental disorder caused by mutations in the VPS51 subunit of the GARP and EARP complexes. Hum. Mol. Genet. 2019, 28, 1548–1560.
- Khakurel, A.; Kudlyk, T.; Lupashin, V.V. Generation and Analysis of hTERT-RPE1 VPS54 Knock-Out and Rescued Cell Lines, In Golgi: Methods and Protocols 2022; Springer: Berlin/Heidelberg, Germany, 2022; pp. 349–364.
- Dell’Angelica, E.C.; Bonifacino, J.S. Coatopathies: Genetic disorders of protein coats. Annu. Rev. Cell Dev. Biol. 2019, 35, 131–168.
- Fröhlich, F.; Petit, C.; Kory, N.; Christiano, R.; Hannibal-Bach, H.K.; Graham, M.; Liu, X.; Ejsing, C.S.; Farese, R.V., Jr.; Walther, T.C. The GARP complex is required for cellular sphingolipid homeostasis. eLife 2015, 4, e08712.
- Takagi, K.; Iwamoto, K.; Kobayashi, S.; Horiuchi, H.; Fukuda, R.; Ohta, A. Involvement of Golgi-associated retrograde protein complex in the recycling of the putative Dnf aminophospholipid flippases in yeast. Biochem. Biophys. Res. Commun. 2012, 417, 490–494.
- Eising, S.; Thiele, L.; Fröhlich, F. A systematic approach to identify recycling endocytic cargo depending on the GARP complex. eLife 2019, 8, e42837.
- O’Brien, C.E.; Younger, S.H.; Jan, L.Y.; Jan, Y.N. The GARP complex prevents sterol accumulation at the trans-Golgi network during dendrite remodeling. J. Cell Biol. 2022, 222, e202112108.
- Hossain, S.; Robbins, N.; Cowen, E.L. The GARP complex is required for filamentation in Candida albicans. Genetics 2022, 222, iyac152.
- Wei, J.; Zhang, Y.-Y.; Luo, J.; Wang, J.-Q.; Zhou, Y.-X.; Miao, H.-H.; Shi, X.-J.; Qu, Y.-X.; Xu, J.; Li, B.-L.; et al. The GARP Complex Is Involved in Intracellular Cholesterol Transport via Targeting NPC2 to Lysosomes. Cell Rep. 2017, 19, 2823–2835.
- Homma, Y.; Fukuda, M. Knockout analysis of Rab6 effector proteins revealed the role of VPS52 in the secretory pathway. Biochem. Biophys. Res. Commun. 2021, 561, 151–157.
- Hirata, T.; Fujita, M.; Nakamura, S.; Gotoh, K.; Motooka, D.; Murakami, Y.; Maeda, Y.; Kinoshita, T. Post-Golgi anterograde transport requires GARP-dependent endosome-to-TGN retrograde transport. Mol. Biol. Cell 2015, 26, 3071–3084.
- Realegeno, S.; Priyamvada, L.; Kumar, A.; Blackburn, J.; Hartloge, C.; Puschnik, A.; Sambhara, S.; Olson, V.; Carette, J.; Lupashin, V.; et al. Conserved Oligomeric Golgi (COG) Complex Proteins Facilitate Orthopoxvirus Entry, Fusion and Spread. Viruses 2020, 12, 707.
- Realegeno, S.; Puschnik, A.S.; Kumar, A.; Goldsmith, C.; Burgado, J.; Sambhara, S.; Olson, V.A.; Carroll, D.; Damon, I.; Hirata, T.; et al. Monkeypox virus host factor screen using haploid cells identifies essential role of GARP complex in extracellular virus formation. J. Virol. 2017, 91, e00011-17.
