Natural fiber (NF) is one of the many resources that nature has provided. NFs decompose quickly and are biodegradable, renewable, and cost-effective. It may be scavenged from a variety of plant and animal sources. They are employed as reinforcing materials in polymers for NF composite development. Because of its environmental friendliness and long-term survivability, NF is growing in appeal among academics and researchers for usage in polymer composites. This study aims to offer a thorough evaluation of the most suitable and widely utilized natural fiber-reinforced polymer composites (NFPCs), along with their manufacture, processing, and applications. It also defines several external treatments of NF and their influence on the characteristics of NFPCs. The characteristics of NFPCs are affected by fiber supply, fiber type, and fiber structure. Numerous physical and chemical treatments were tested to see how they affected the thermal and strength properties of natural fiber-reinforced thermoplastic and thermosetting composites. Several polymer composite fabrication techniques were also studied. NFPCs have several disadvantages, notably low fire protection, poor strength properties, and greater moisture absorption, which have prevented their application. It is shown how NFPCs are employed in a variety of industries, particularly automotive and research industries. The review discovered that intentionally changing the regular fiber enhanced the thermochemical and physico-mechanical properties of the NFPCs by means of improving the grip between the fiber surface and the polymer framework. This study aims to provide important and fundamental facts on NF and their composites, which will aid in new investigations, the creation of a creative framework for polymer composite types, and the achievement of Sustainable Development Goals.
- agro-waste
- epoxy
- fabrication techniques
- natural fiber
- polymer composite
- surface modification
1. Modifications in Physical Treatment
2. Chemical Treatments
2.1. Acetylation Treatment
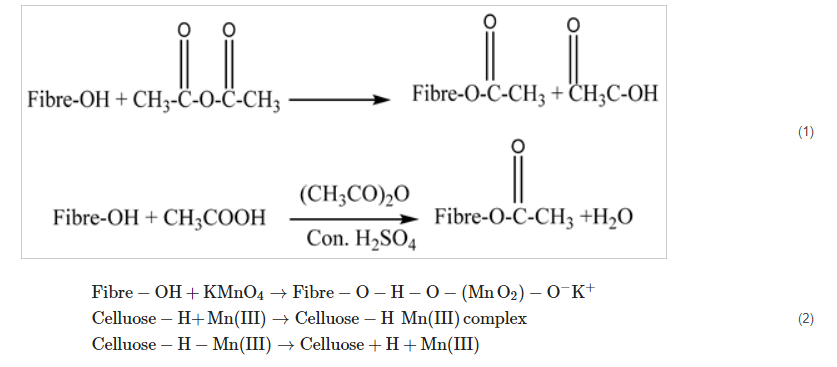
2.2. Acrylation Treatment
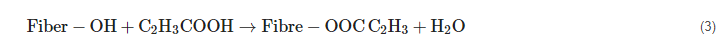
2.3. Grafting Using Acrylonitrile
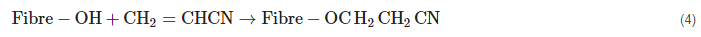
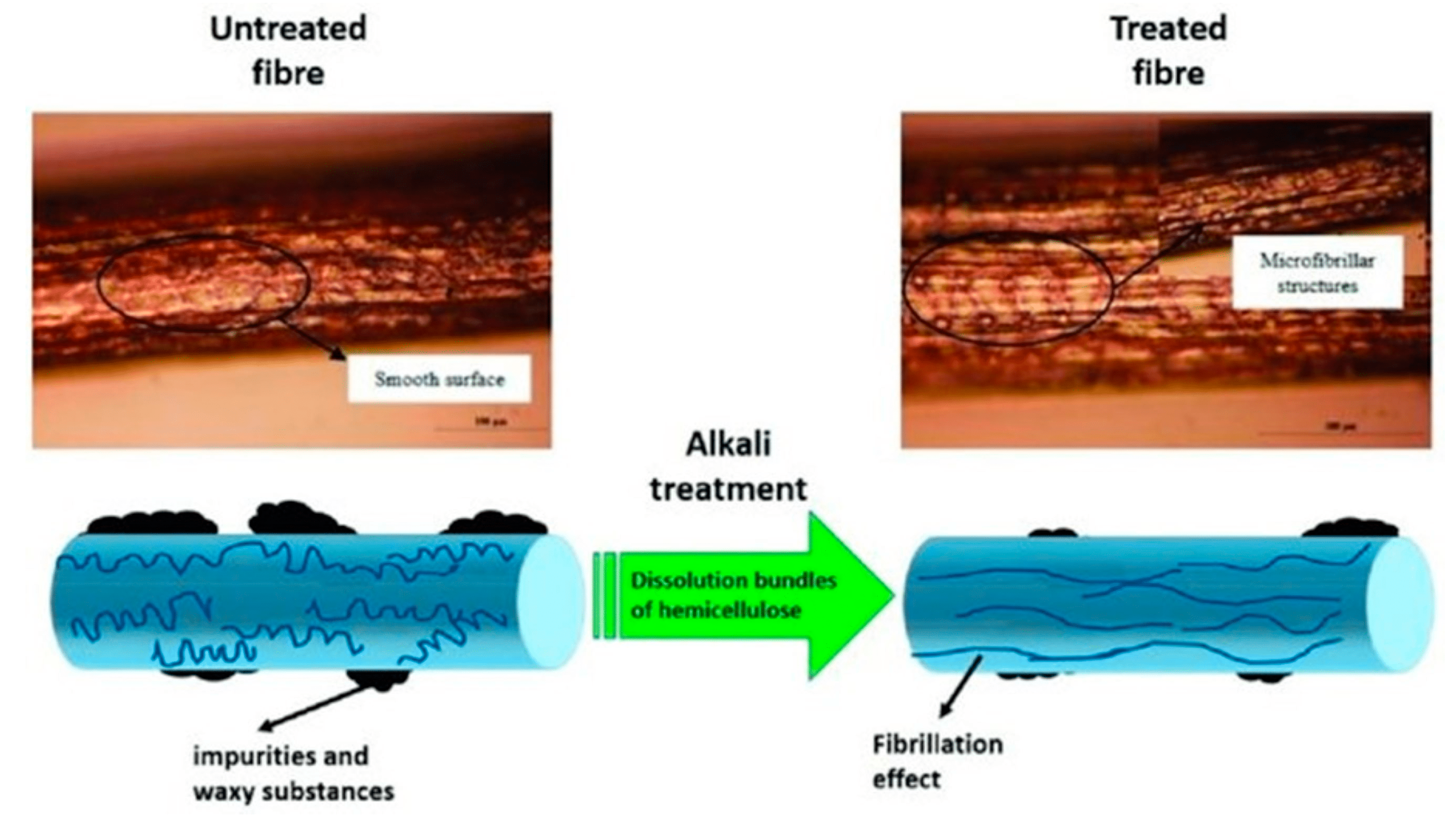
2.4. Alkaline Treatment
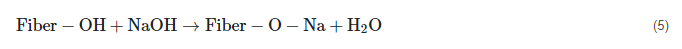
2.5. Benzoylation Treatment
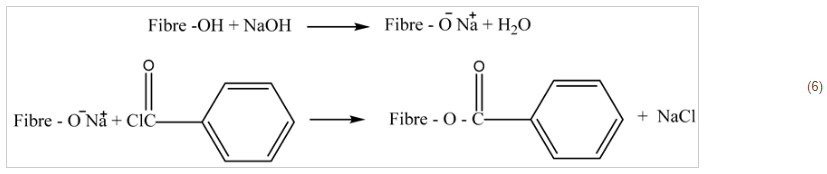
2.6. Etherification
2.7. Treatment with Isocyanates

2.8. Permanganate Treatment
2.9. Maleated Coupling Agents
2.10. Treatment with Peroxide



2.11. Silane Treatment
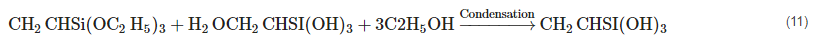
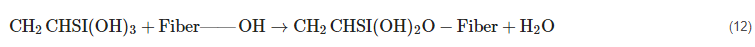
3. Biological (Fungi and Enzymes)
References
- Zhou, Y.; Fan, M.; Chen, L. Interface and bonding mechanisms of plant fibre composites: An overview. Compos. Part B Eng. 2016, 101, 31–45.
- Ferreira, D.P.; Cruz, J.; Fangueiro, R. Surface modification of natural fibers in polymer composites. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netheralnds, 2019; pp. 3–41.
- Yaro, N.S.A.; Sutanto, M.H.; Habib, N.Z.; Napiah, M.; Usman, A.; Muhammad, A. Comparison of Response Surface Methodology and Artificial Neural Network approach in predicting the performance and properties of palm oil clinker fine modified asphalt mixtures. Constr. Build. Mater. 2022, 324, 126618.
- De Araujo Alves Lima, R.; Kawasaki Cavalcanti, D.; de Souza e Silva Neto, J.; Meneses da Costa, H.; Banea, M.D. Effect of surface treatments on interfacial properties of natural intralaminar hybrid composites. Polym. Compos. 2020, 41, 314–325.
- Verma, A.; Parashar, A.; Jain, N.; Singh, V.; Rangappa, S.M.; Siengchin, S. Surface Modification techniques for the preparation of different novel biofibers for composites. In Biofibers and Biopolymers for Biocomposites; Springer: Berlin/Heidelberg, Germany, 2020; p. 134.
- Kankia, M.U.; Baloo, L.; Danlami, N.; Samahani, W.N.; Mohammed, B.S.; Haruna, S.; Jagaba, A.H.; Abubakar, M.; Ishak, E.A.; Sayed, K. Optimization of Cement-Based Mortar Containing Oily Sludge Ash by Response Surface Methodology. Materials 2021, 14, 6308.
- George, M.; Mussone, P.G.; Abboud, Z.; Bressler, D.C. Characterization of chemically and enzymatically treated hemp fibres using atomic force microscopy and spectroscopy. Appl. Surf. Sci. 2014, 314, 1019–1025.
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2018, 47, 2153–2183.
- Jagaba, A.H.; Kutty, S.R.M.; Lawal, I.M.; Aminu, N.; Noor, A.; Al-Dhawi, B.N.S.; Usman, A.K.; Batari, A.; Abubakar, S.; Birniwa, A.H.; et al. Diverse sustainable materials for the treatment of petroleum sludge and remediation of contaminated sites: A review. Clean. Waste Syst. 2022, 2, 100010.
- Bledzki, A.K.; Mamun, A.A.; Lucka-Gabor, M.; Gutowski, V.S. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008, 2, 413–422.
- Mahesha, G.; Shenoy, S.B.; Kini, V.M.; Padmaraja, N. Effect of fiber treatments on mechanical properties of Grewia serrulata bast fiber reinforced polyester composites. Mater. Today Proc. 2018, 5, 138–144.
- Amiandamhen, S.O.; Meincken, M.; Tyhoda, L. The effect of chemical treatments of natural fibres on the properties of phosphate-bonded composite products. Wood Sci. Technol. 2018, 52, 653–675.
- Liu, Z.; Tisserat, B.H. Coating applications to natural fiber composites to improve their physical, surface and water absorption characters. Ind. Crop. Prod. 2018, 112, 196–199.
- Kadem, S.; Irinislimane, R.; Belhaneche-Bensemra, N. Novel Biocomposites Based on Sunflower Oil and Alfa Fibers as Renewable Resources. J. Polym. Environ. 2018, 26, 3086–3096.
- Patel, V.; Parsania, P. Performance Evaluation of Alkali and Acrylic Acid Treated—Untreated Jute Composites of Mixed Epoxy—Phenolic Resins. J. Reinf. Plast. Compos. 2010, 29, 725–730.
- Jagaba, A.; Kutty, S.; Hayder, G.; Baloo, L.; Noor, A.; Yaro, N.; Saeed, A.; Lawal, I.; Birniwa, A.; Usman, A. A Systematic Literature Review on Waste-to-Resource Potential of Palm Oil Clinker for Sustainable Engineering and Environmental Applications. Materials 2021, 14, 4456.
- Birniwa, A.H.; Abdullahi, S.S. Study on physico-mechanical behaviour of acacia nilotica (gum tree) and glass fiber blend reinforced epoxy resin composite. ChemSearch J. 2019, 10, 46–53.
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Zakaria, Z.Y.; Jagaba, A.H.; Ghaleb, A.A.S.; Mohammed, H.G. Pristine and magnetic kenaf fiber biochar for Cd2+ adsorption from aqueous solution. Int. J. Environ. Res. Public Health 2021, 18, 7949.
- Singha, A.S.; Raan, R.K. Chemically induced graft copolymerization of acrylonitrile onto lignocellulosic fibers. Appl. Polym. 2012, 124, 1891–1898.
- Kenned, J.J.; Sankaranarayanasamy, K.; Kumar, C.S. Chemical, biological, and nanoclay treatments for natural plant fiber-reinforced polymer composites: A review. Polym. Polym. Compos. 2020, 29, 1011–1038.
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646.
- Aseer, J.R.; Sankaranarayanasamy, K.; Jayabalan, P.; Natarajan, R.; Dasan, K.P. Morphological, Physical, and Thermal Properties of Chemically Treated Banana Fiber. J. Nat. Fibers 2013, 10, 365–380.
- Rangaraj, R.; Sathish, S.; Mansadevi, T.L.D.; Supriya, R.; Surakasi, R.; Aravindh, M.; Karthick, A.; Mohanavel, V.; Ravichandran, M.; Muhibbullah, M.; et al. Investigation of Weight Fraction and Alkaline Treatment on Catechu Linnaeus/Hibiscus cannabinus/Sansevieria Ehrenbergii Plant Fibers-Reinforced Epoxy Hybrid Composites. Adv. Mater. Sci. Eng. 2022, 2022, 1–9.
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33.
- Rajesh, G.; Prasad, A.V.R. Tensile properties of successive alkali treated short jute fiber reinforced PLA composites. Procedia Mater. Sci. 2014, 5, 2188–2196.
- Vijay, R.; Singaravelu, D.L.; Vinod, A.; Sanjay, M.; Siengchin, S.; Jawaid, M.; Khan, A.; Parameswaranpillai, J. Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens. Int. J. Biol. Macromol. 2019, 125, 99–108.
- Ilyas, R.; Sapuan, S.; Ishak, M.; Zainudin, E. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388.
- Sathish, S.; Karthi, N.; Prabhu, L.; Gokulkumar, S.; Balaji, D.; Vigneshkumar, N.; Farhan, T.A.; AkilKumar, A.; Dinesh, V. A review of natural fiber composites: Extraction methods, chemical treatments and applications. Mater. Today Proc. 2021, 45, 8017–8023.
- Jagaba, A.H.; Kutty, S.R.M.; Isa, M.H.; Ghaleb, A.A.S.; Lawal, I.M.; Usman, A.K.; Birniwa, A.H.; Noor, A.; Abubakar, S.; Umaru, I.; et al. Toxic Effects of Xenobiotic Compounds on the Microbial Community of Activated Sludge. ChemBioEng Rev. 2022, 9, 497–535.
- Jagaba, A.H.; Kutty, S.R.M.; Lawal, I.M.; Birniwa, A.H.; Affam, A.C.; Yaro, N.S.A.; Usman, A.K.; Umaru, I.; Abubakar, S.; Noor, A. Circular economy potential and contributions of petroleum industry sludge utilization to environmental sustainability through engineered processes—A review. Clean. Circ. Bioecon. 2022, 3, 100029.
- Nayak, S.; Mohanty, J.R. Influence of chemical treatment on tensile strength, water absorption, surface morphology, and thermal analysis of areca sheath fibers. J. Nat. Fibers 2019, 16, 589–599.
- Abd Halip, J.; Hua, L.S.; Ashaari, Z.; Tahir, P.M.; Chen, L.W.; Uyup, M.K.A. Effect of treatment on water absorption behavior of natural fiber–Reinforced polymer composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–156.
- Abdul Majid, R.; Ismail, H.; Mat Taib, R. Engineering, Processing, tensile, and thermal studies of poly (vinyl chloride)/epoxidized natural rubber/kenaf core powder composites with benzoyl chloride treatment. Polym.-Plast. Technol. Eng. 2018, 57, 1507–1517.
- Swain, P.T.R.; Biswas, S. A comparative analysis of physico-mechanical, water absorption, and morphological behaviour of surface modified woven jute fiber composites. Polym. Compos. 2018, 39, 2952–2960.
- Prabhu, L.; Krishnaraj, V.; Sathish, S.; Gokulkumar, S.; Karthi, N.; Rajeshkumar, L.; Balaji, D.; Vigneshkumar, N.; Elango, K. A review on natural fiber reinforced hybrid composites: Chemical treatments, manufacturing methods and potential applications. Mater. Today Proc. 2021, 45, 8080–8085.
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2020, 55, 829–892.
- Albdiry, M.T.; Yousif, B.; Ku, H.; Lau, K.T. A critical review on the manufacturing processes in relation to the properties of nanoclay/polymer composites. J. Compos. Mater. 2013, 47, 1093–1115.
- Rowell, R.M.; Chen, G.C. Epichlorohydrin coupling reactions with wood. Wood Sci. Technol. 1994, 28, 371–376.
- Lawal, I.M.; Bertram, D.; White, C.J.; Jagaba, A.H.; Hassan, I.; Shuaibu, A. Multi-criteria performance evaluation of gridded precipitation and temperature products in data-sparse regions. Atmosphere 2021, 12, 1597.
- Jagaba, A.H.; Kutty, S.R.M.; Noor, A.; Isah, A.S.; Lawal, I.M.; Birniwa, A.H.; Usman, A.K.; Abubakar, S. Kinetics of Pulp and Paper Wastewater Treatment by High Sludge Retention Time Activated Sludge Process. J. Hunan Univ. Nat. Sci. 2022, 49, 242–251.
- Hassan, M.L.; El-Wakil, N.A.; Sefain, M.Z. Thermoplasticization of bagasse by cyanoethylation. J. Appl. Polym. Sci. 2001, 79, 1965–1978.
- Sefain, M.Z.; Fadl, M.H.; Elwakil, N.A.; Naoum, M.M. Kinetics of heterogeneous cyanoethylation of cellulose. Polym. Int. 1993, 32, 251–255.
- Jagaba, A.H.; Kutty, S.R.M.; Salih, G.H.A.; Noor, A.; HAFIZ, M.; Yaro, N.S.A.; Saeed, A.A.H.; Lawal, I.M.; Birniwa, A.H.; Kilaco, A.U. Palm Oil Clinker as a Waste By-Product: Utilization and Circular Economy Potential; IntechOpen: London, UK, 2021; Volume 1.
- Liu, W.; Qiu, R.; Li, K. Effects of fiber extraction, morphology, and surface modification on the mechanical properties and water absorption of bamboo fibers-unsaturated polyester composites. Polym. Compos. 2016, 37, 1612–1619.
- Yaro, N.S.A.; Napiah, M.; Sutanto, M.H.; Usman, A.; Jagaba, A.H.; Umar, A.M.; Ahmad, A. Geopolymer utilization in the pavement industry-An overview. In Proceedings of the 6th International Conference on Civil and Environmental Engineering for Sustainability (IConCEES 2021), Online, 15–16 November 2021; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; p. 012025.
- Paul, A.; Joseph, K.; Thomas, S. Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforced with short sisal fibers. Compos. Sci. Technol. 1997, 57, 67–79.
- Yaro, N.S.A.; Sutanto, M.H.; Habib, N.Z.; Napiah, M.; Usman, A.; Jagaba, A.H.; Al-Sabaeei, A.M. Application and circular economy prospects of palm oil waste for eco-friendly asphalt pavement industry: A review. J. Road Eng. 2022, 2, 309–331.
- Abdullahi, S.S.; Musa, H.; Habibu, S.; Birniwa, A.H.; Mohammad, R.E.A. Facile synthesis and dyeing performance of some disperse monomeric and polymeric dyes on nylon and polyester fabrics. Bull. Chem. Soc. Ethiop. 2022, 35, 485–497.
- Birniwa, A.H.; Kehili, S.; Ali, M.; Musa, H.; Ali, U.; Kutty, S.R.M.; Jagaba, A.H.; Abdullahi, S.S.; Tag-Eldin, E.M.; Mahmud, H.N.M.E. Polymer-Based Nano-Adsorbent for the Removal of Lead Ions: Kinetics Studies and Optimization by Response Surface Methodology. Separations 2022, 9, 356.
- He, L.; Li, W.; Chen, D.; Lu, G.; Chen, L.; Zhou, D.; Yuan, J. Investigation on the microscopic mechanism of potassium permanganate modification and the properties of ramie fiber/polypropylene composites. Polym. Compos. 2018, 39, 3353–3362.
- Mohammed, A.A.; Bachtiar, D.; Rejab, M.R.M.; Jiang, X.X.; Abas, F.O.; Abass, R.U.; Hasany, S.F.; Siregar, J.P. Effects of KMnO4 Treatment on the Flexural, Impact, and Thermal Properties of Sugar Palm Fiber-Reinforced Thermoplastic Polyurethane Composites. JOM 2018, 70, 1326–1330.
- Birniwa, A.H.; Mahmud, H.N.M.E.; Abdullahi, S.S.; Habibu, S.; Jagaba, A.H.; Ibrahim, M.N.M.; Ahmad, A.; Alshammari, M.B.; Parveen, T.; Umar, K. Adsorption Behavior of Methylene Blue Cationic Dye in Aqueous Solution Using Polypyrrole-Polyethylenimine Nano-Adsorbent. Polymers 2022, 14, 3362.
- Sayed, K.; Baloo, L.; Kutty, S.R.B.; Al Madhoun, W.; Kankia, M.U.; Jagaba, A.H.; Singa, P.K. Optimization of palm oil mill effluent final discharge as biostimulant for biodegradation of tapis light crude petroleum oil in seawater. J. Sea Res. 2022, 188, 102268.
- Daghigh, V.; Lacy, T.E.; Pittman, C.U.; Daghigh, H. Influence of maleated polypropylene coupling agent on mechanical and thermal behavior of latania fiber-reinforced PP/EPDM composites. Polym. Compos. 2018, 39, E1751–E1759.
- Huang, C.-W.; Yang, T.-C.; Wu, T.-L.; Hung, K.-C.; Wu, J.-H. Effects of maleated polypropylene content on the extended creep behavior of wood–polypropylene composites using the stepped isothermal method and the stepped isostress method. Wood Sci. Technol. 2018, 52, 1313–1330.
- Keener, T.; Stuart, R.; Brown, T. Maleated coupling agents for natural fibre composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 357–362.
- Birniwa, A.H.; Salisu, A.A.; Musa, H. Dyeing and antimicrobial activity of acacia nilotica (linnaeus) natural colourant extract on cotton fabric material against selected gram (-) and gram (+) bacteria. Acta Biol. Malays. 2014, 3, 77–83.
- Al-dhawi, B.N.S.; Kutty, S.R.M.; Ghaleb, A.A.S.; Almahbashi, N.M.Y.; Saeed, A.A.H.; Al-Mekhlafi, A.B.A.; Alsaeedi, Y.A.A.; Jagaba, A.H. Pretreated palm oil clinker as an attached growth media for organic matter removal from synthetic domestic wastewater in a sequencing batch reactor. Case Stud. Chem. Environ. Eng. 2022, 7, 100294.
- Vimalanathan, P.; Venkateshwaran, N.; Srinivasan, S.P.; Santhanam, V.; Rajesh, M. Impact of surface adaptation and Acacia nilotica biofiller on static and dynamic properties of sisal fiber composite. Int. J. Polym. Anal. Charact. 2018, 23, 99–112.
- Wong, J.Y.M.; Chan, M.Y. Influence of bleaching treatment by hydrogen peroxide on chitosan/durian husk cellulose biocomposite films. Adv. Polym. Technol. 2018, 37, 2462–2469.
- Aguilar-Rios, A.; Herrera-Franco, P.; Martinez-Gomez, A.D.J.; Valadez-Gonzalez, A. Improving the bonding between henequen fibers and high density polyethylene using atmospheric pressure ethylene-plasma treatments. Express Polym. Lett. 2014, 8, 491–504.
- Agrawal, R.; Saxena, N.; Sharma, K.; Thomas, S.; Sreekala, M. Activation energy and crystallization kinetics of untreated and treated oil palm fibre reinforced phenol formaldehyde composites. Mater. Sci. Eng. A 2000, 277, 77–82.
- Seki, Y. Innovative multifunctional siloxane treatment of jute fiber surface and its effect on the mechanical properties of jute/thermoset composites. Mater. Sci. Eng. A 2009, 508, 247–252.
- Balarabe, S.; Habibu, S.; Gumel, S.M.; Ladan, M.; Haruna, A. Dyeing and antibacterial finishing of cotton fabric using Diospyros mespiliformis leaves extracts. Malays. J. Fund. Appl. Sci. 2017, 13, 175–178.
- Jagaba, A.H.; Kutty, S.R.M.; Isa, M.H.; Affam, A.C.; Aminu, N.; Abubakar, S.; Noor, A.; Lawal, I.M.; Umaru, I.; Hassan, I. Effect of environmental and operational parameters on sequential batch reactor systems in dye degradation. In Dye Biodegradation, Mechanisms and Techniques; Springer: Berlin/Heidelberg, Germany, 2022; pp. 193–225.
- Atiqah, A.; Jawaid, M.; Ishak, M.R.; Sapuan, S.M. Effect of Alkali and Silane Treatments on Mechanical and Interfacial Bonding Strength of Sugar Palm Fibers with Thermoplastic Polyurethane. J. Nat. Fibers 2018, 15, 251–261.
- Sani, S.; Kurawa, M.A.; Sira, I.T.; Birniwa, A.H.; Zauro, S.A. Liquid-assisted Mechanochemical Conversion of 2-hydroxy-3-methoxybenzaldehyde and Some Primary Aromatic Amines to Corresponding Schiff bases. ChemSearch J. 2018, 9, 1–7.
