The features of allergic asthma are believed to be mediated mostly through the Th2 immune response. In this Th2-dominant concept, the airway epithelium is presented as the helpless victim of Th2 cytokines. Asthma researchers started believing in that the airway epithelium played a crucial role, as alarmins, which are the inducers of type 2 innate lymphoid cell (ILC2), are almost exclusively secreted by the airway epithelium. This underscores the eminence of airway epithelium in asthma pathogenesis. However, the airway epithelium has a bipartite functionality in sustaining healthy lung homeostasis and asthmatic lungs. On the one hand, the airway epithelium maintains lung homeostasis against environmental irritants/pollutants with the aid of its various armamentaria, including its chemosensory apparatus and detoxification system. Alternatively, it induces an ILC2-mediated type 2 immune response through alarmins to amplify the inflammatory response. However, the available evidence indicates that restoring epithelial health may attenuate asthmatic features.
- allergic asthma
- airway epithelium
- alarmins
1. Barrier Function of Airway Epithelium
1.1. Importance of Epithelial Barrier in Maintaining Homeostasis
1.2. Anatomical Barrier Role of Airway Epithelium
Epithelial junctional complexes act as crucial signaling hubs and threat detectors, interacting with the microenvironment and monitoring self-defense. This function of the epithelial barrier of the bronchial epithelial cells and its structural integrity is mainly conferred by the adhesive forces of the intercellular junctions. The major intercellular junction proteins documented in preserving the barrier include tight junctions, the adherens junctions, and the (hemi) desmosomes [4]. The tight and adherens junctions together form the apical junctional complex (AJC) and are present at the apicolateral border of the airway epithelial cells (Figure 1).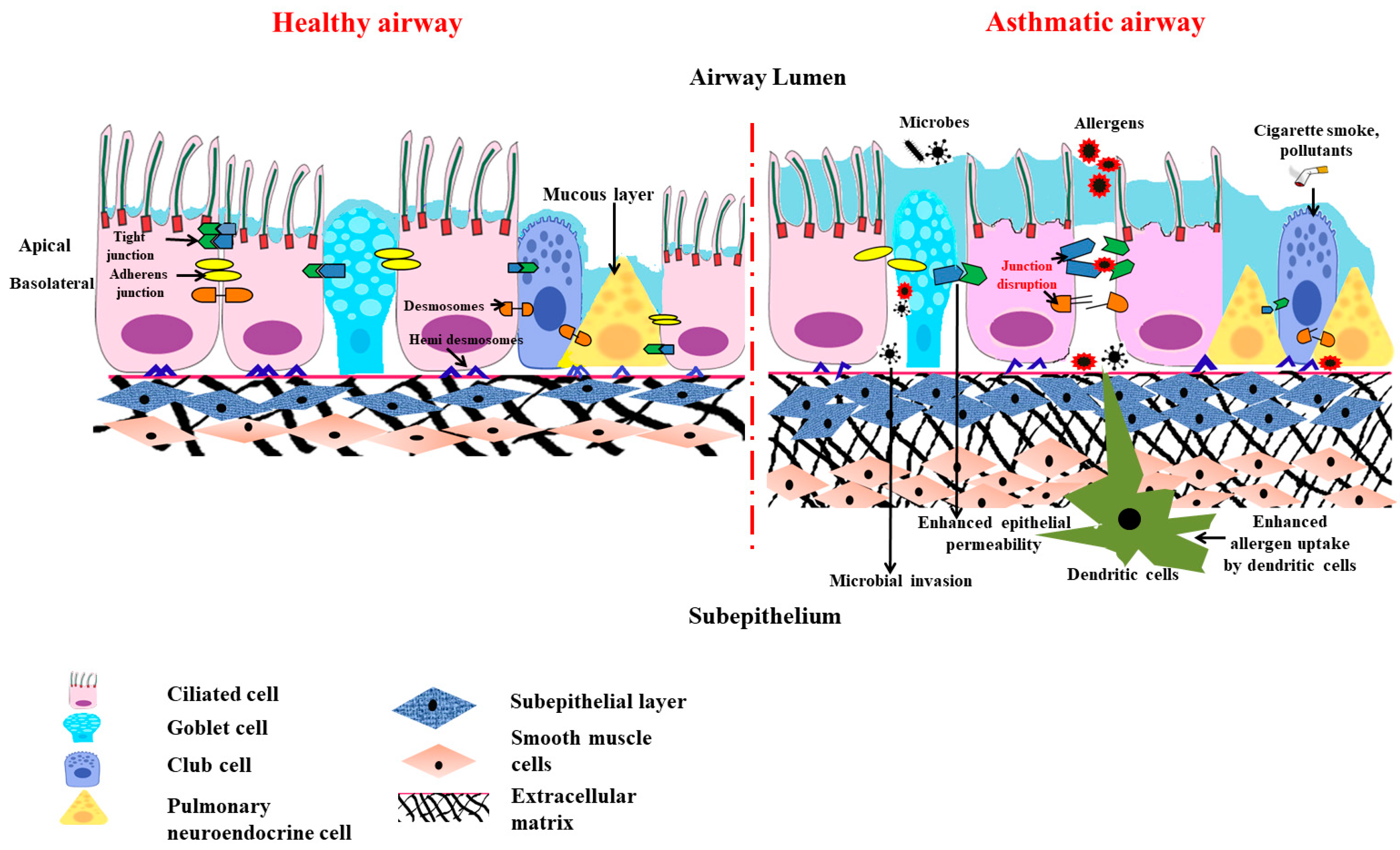
1.3. Chemical Barrier Role of Airway Epithelium
1.4. Physiological Barrier in Airway
The airway defense responses that guard the lungs and the rest of the body against inhaled irritants like cigarette smoke and aerosols include bronchoconstriction, which is a crucial and effective reflex mechanism [16]. However, the chemical irritants stimulate the sensory nerves present in the respiratory tract, and the generated action potential is conducted by the vagus nerves (to the brain stem). This instantly causes bronchoconstriction via the cholinergic efferent pathway accompanied by the hypersecretion of mucus, coughing, and dyspneic sensations [17]. Thereof, the conjecture that bronchoconstriction is a physiological protective mechanism, becomes a clinical symptom as this also confines the entry of the air and, thus, difficulty in breathing occurs subsequent to the bronchoconstriction.1.5. Special Cellular Machinery in Airway Epithelial Barrier
Surprisingly, airway epithelial layers also have special machinery, like pulmonary neuroendocrine cells (PNECs), that produce an array of neuropeptides, neurotransmitters, and amines to sense the environmental air and catabolize the irritants/toxicants of the air to neutralize them [18][19]. Therefore, these cells are considered intrapulmonary sensors that also sense hypoxia by chemoreception [20]. Indeed, like the traditional chemoreceptors present in the nose, the PNEC clusters that are part of the epithelial layer have been shown to have olfactory receptors so that they can sense the toxicants in the inspired air and react to prevent the further entry of such toxicants through induction of bronchoconstriction [21].1.6. Barrier Function of Airway Epithelium against Air Pollutants and Pathogens
Air pollutants are typically categorized as ultrafine, fine, and coarse, depending on their size, source, and nature (gases or particles). The main sources of indoor air pollution include stoves, biological substances (including mold), microplastics, and household dust, whereas automobile, industrial (urban), and agricultural (rural) activity are the major reason for outdoor pollution [22]. Airborne particulate matter (PM) is a heterogeneous mixture of solids and aerosols, which can include heavy metals, airborne dust, and nanoparticles discharged from chemical factories, wildfire smoke, vehicle exhaust, and volcanic eruptions. Urban homes have PM2.5- and PM10-rich indoor pollutants along with higher NO2 levels [23]. Since fine PM penetrates the narrow airways more deeply than coarse PM, it is particularly dangerous to breathe it in. Inhalation of fine particulates has been linked to the development of asthma and COPD. A correlation has been put forward between increased levels of outdoor pollution, particularly NO2, PM2.5, and black carbon, and the onset and development of childhood asthma along with lowered lung function [24]. In addition to pollutants and allergens, pathogens also have a major role in disrupting the epithelial barrier. Adivitiya et al. have shown the impact Sars Cov2 has on mucociliary clearance through protein network analysis. The spike (S) protein of the virus utilizes ACE2 (angiotensin-converting enzyme 2) and TMPRSS2 protease, which are present in the ciliated and secretory cells, to enter the host. The receptor-binding domain of S also interacts with CD209, a lectin protein found in the epithelial cell, to facilitate virus entry.2. The Victim Role of Airway Epithelium in Asthma Pathogenesis
2.1. Role of Th2 Cytokines in Airway Epithelial Barrier Dysfunction
It is generally believed that T helper 2 lymphocytes play a crucial role in asthma development after the initial sensitization phase (with allergen exposure). Upon repeated secondary exposures to the same allergen, Th2 cells accumulate in the lungs, and the features of allergic asthma develop. This is popularly referred to as type2/Th2 asthma, whereby such Th2 cells release various cytokines, like IL-4, IL-5, and IL-13, in response to allergens [25]. (a) IL-4 and IL-13: Both IL4 and IL-13 are crucial Th2 cytokines responsible for immunoglobulin class switching to enhance IgE production [26], leading to the degranulation of mast cells and basophils. The released proinflammatory meditators not only caused bronchoconstriction, but also cause airway epithelial injury. Treatment with both IL-4 and IL-13 cytokines showed reduced expression in the apical junctional complex proteins that encompass both the tight junction and adherent junction [27]. Treatment with IL-4 in HBEC (human bronchial epithelial cells) increases permeability in epithelial cells. There is reduced transepithelial electrical resistance that, in turn, leads to increased allergen sensitization and allergen uptake. IL-4 and IL-13 also cause a reduction in the ciliary beating of the ciliated epithelial cells and, in turn, in mucociliary clearance [28]. IL-13 also plays a major role in the secretion of periostin (POSTN), which is an essential biomarker in asthma. Periostin is an extracellular protein present in the matrix. It is a downstream product of the IL-13 pathway, signifying type 2 immunity. POSTN release is then coupled with epithelial mesenchyme transition, which is also shown in vitro in Beas2B cell lines. Thus, POSTN plays a major role in airway remodeling [29]. Overall, these cytokines are responsible for injuries to the airway epithelium. In addition to causing airway epithelial injury through oxidative stress, both IL-4 and IL-13 have been shown to cause perturbation in airway epithelial integrity with barrier dysfunction [27][30]. (b) IL-5: IL-5 plays a crucial role in differentiation, activation, maturation, and the recruitment of eosinophil that releases major basic cationic proteins, like major basic protein-1 (MBP-1), eosinophil cationic protein (ECP), eosinophil derived neurotoxin (EDN), and eosinophil peroxidase (EPO-1), that induce oxidative stress, causing an injury to the airway epithelium [31].2.2. Mitochondrial Dysfunction in Asthmatic Airway Epithelium
The earlier clinical study found the presence of an increased number of mitochondria with altered structures in asthmatic children [32]. Later, a lab demonstrated the involvement of mitochondrial dysfunction in asthma pathogenesis [33]. The lab also demonstrated a reduction in the cytochrome c oxidase (COX), which is the primary enzyme of the electron transport chain (ETC) residing in mitochondria’s inner mitochondrial membrane (IMM), which transfers electrons from the cytochrome c to oxygen in the lungs of mice with allergic airway inflammation.
While it is known that both IL-4 and IL-13 promote IgE class switching through STAT-6, they also induce an enzyme: 12/15-lipoxygenase (12/15-LOX, also called 15-lipooxygenase in humans) [34]. Even though the role of 5-lipoxygenase (5-LOX) is well established in asthma pathogenesis, the detailed role of 12/15-lipoxygenase was demonstrated. 12/15-LOX is one of the enzymes responsible for cellular suicide via the programmed disappearance of mitochondria and other cellular organelles from the reticulocytes and for immature fibroblasts converting into red blood cells and mature fibroblasts, respectively [35][36][37]. This is essential for the uninterrupted functions of red blood cells and mature fibroblasts, as the presence of organelles in these cells disturbs their functions, such as effective gas exchange and clear vision, respectively. Though it was known earlier that 12/15-LOX was increased in asthmatic lungs [38][39], its role in mitochondrial dysfunction was not known. The mere overexpression of 12/15-LOX in naïve mice causes mitochondrial dysfunction, along with the development of asthma-like features, indicating the pathogenetic role of 12/15-LOX [40].
3. Governing/Immune Role of Airway Epithelium in Allergic Airway Inflammation
3.1. Less Dominant Role of Inflammation in Causing Epithelial Barrier Dysfunction
The Th2 immune response has been demonstrated as the causative factor for inciting the loss of epithelial layer integrity. However, numerous pieces of literature also suggest the possibility of inflammation-independent epithelial cell dysfunction. Numerous asthma susceptibility genes, for instance, IL33, IL1RL1, MUC5AC, TSLP, CDHR3, and KIF3A, are expressed in the airway epithelium. This highlights the significance of the airway epithelium in the development of asthma [41]. Anomalies in the epithelial barrier due to disruptions in the tight and adherens junctions have been proclaimed to be involved in allergen sensitization and asthma advancement [42]. All these indicate that asthmatic people might have a compromised and dysfunctional epithelial barrier. Genome-wide association studies in asthmatics have shown the association of various genes with asthma susceptibility and how these genes are also expressed in the airway epithelium [43]. In addition to the inherent barrier defects seen in asthma patients, allergens directly affect the lung epithelial barrier, the first layer of defense in them. Allergens, such as house dust mites (HDMs), pollens, cockroach extracts, and fungi, produces or contain proteases and hence disturb the epithelial barrier, causing increased sensitization [44]. Inhaled allergens or proteases can cause epithelial cells to recognize and react by activating a variety of PRRs, including TLR and PAR. NF-κB activation is stirred up by these activated receptor signals. This, in turn, causes transcriptional activation of myriads’ pro-inflammatory genes, including cytokines and chemokines. As the role of NOD-like receptors in allergic inflammation is complex and context-dependent [45], the role of innate immune receptors in allergic inflammation is complex.3.2. Less Dominant Role of Inflammation in Causing Mitochondrial Dysfunction in Airway Epithelia
Similar to epithelial barrier dysfunction, mitochondrial dysfunction in asthmatic airway epithelia can additionally be unaided by inflammation. Investigations and research have already determined the possibility of inflammation and oxidative stress-induced mitochondrial dysfunction in asthmatic airway epithelia. When there was a forced reduction in the expression of certain ETC enzymes in airway epithelium, allergic airway inflammation features got developed [46]. This indicates the possibility of inflammation-independent mitochondrial dysfunction in asthma pathogenesis.3.3. Airway Epithelium Induces ILC2-Mediated Type 2 Immune Response through Alarmins
So far, the conventional rationale about the airway epithelium is of a ‘victim’ that often comes under recurrent stress from the inflammatory system in asthma. Albeit, over the last decade, mounting evidence has shifted the paradigm for the role of airway epithelium towards a more upstream modulator in airway inflammation. Several PRRs, including TLR2, TLR4, NOD1 [47][48], and PAR1-4, are expressed by airway epithelial cells [49][50]. Upon stimulation, they activate various signaling pathways that lead to the enlistment of immune cells and also the Th2 immune response [51]. One major group of cytokines which is recognized as important in this epithelium-driven immune response is called ‘alarmins’ [52]. ‘Alarmins’, specifically thymic stromal lymphopoietin (TSLP), interleukin-33, and interleukin-25, are released by airway epithelium upon cellular stress or damage caused by an allergen, pathogen, or pollutant exposure ultimately skews the immune response toward type 2 [53][54]. The concept of alarmins was introduced by Joost J Oppenheim. He used this umbrella term to describe a group of host proteins that are released upon cell damage or pathogen challenge, which recruit and activate both innate and adaptive immunity and galvanize the whole immune response through ‘early signals’ [52]. Although airway epithelium is considered the major source of alarmins but innate, adaptive, and other structural cells can also secrete alarmins [55] (Figure 2).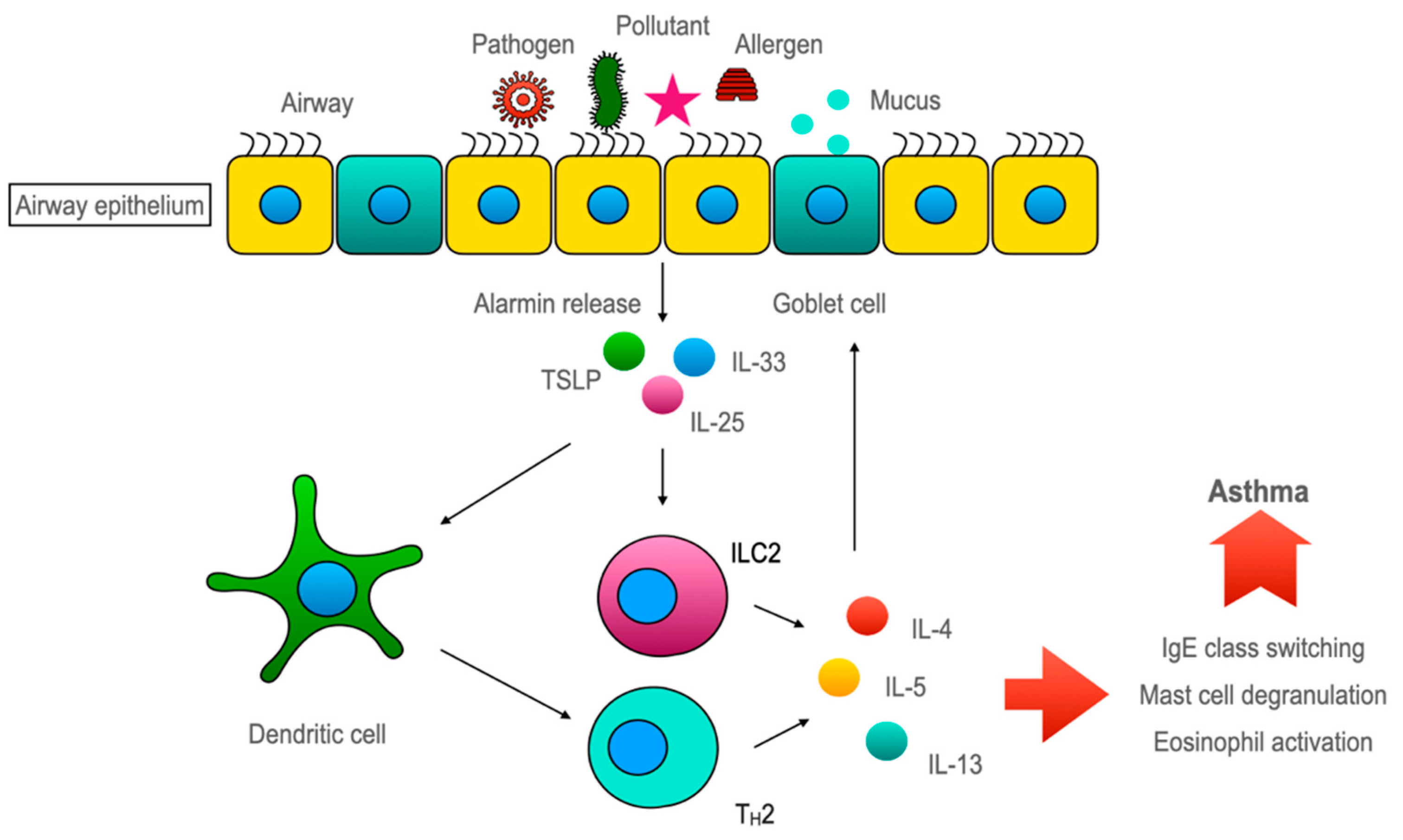
64. Conclusions
References
- Sparr, E.; Millecamps, D.; Isoir, M.; Burnier, V.; Larsson, Å.; Cabane, B. Controlling the hydration of the skin though the application of occluding barrier creams. J. R. Soc. Interface 2012, 10, 20120788.
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573.
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520.
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669.
- Overgaard, C.E.; Mitchell, L.A.; Koval, M. Roles for claudins in alveolar epithelial barrier function. Ann. N. Y. Acad. Sci. 2012, 1257, 167–174.
- Soini, Y. Claudins in lung diseases. Respir. Res. 2011, 12, 70.
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflug. Arch 2017, 469, 135–147.
- Niessen, C.M.; Gottardi, C.J. Molecular components of the adherens junction. Biochim. Biophys. Acta 2008, 1778, 562–571.
- Fu, R.; Jiang, X.; Li, G.; Zhu, Y.; Zhang, H. Junctional complexes in epithelial cells: Sentinels for extracellular insults and intracellular homeostasis. FEBS J. 2022, 289, 7314–7333.
- Mitamura, Y.; Ogulur, I.; Pat, Y.; Rinaldi, A.O.; Ardicli, O.; Cevhertas, L.; Brüggen, M.C.; Traidl-Hoffmann, C.; Akdis, M.; Akdis, C.A. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermat. 2021, 85, 615–626.
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632.
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35.
- Kaushik, M.S.; Chakraborty, S.; Veleri, S.; Kateriya, S. Mucociliary Respiratory Epithelium Integrity in Molecular Defense and Susceptibility to Pulmonary Viral Infections. Biology 2021, 10, 95.
- Zhang, N.; Van Crombruggen, K.; Gevaert, E.; Bachert, C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy 2016, 71, 295–307.
- Leiva-Juárez, M.M.; Kolls, J.K.; Evans, S.E. Lung epithelial cells: Therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018, 11, 21–34.
- Gu, X.; Karp, P.H.; Brody, S.L.; Pierce, R.A.; Welsh, M.J.; Holtzman, M.J.; Ben-Shahar, Y. Chemosensory functions for pulmonary neuroendocrine cells. Am. J. Respir. Cell Mol. Biol. 2014, 50, 637–646.
- Gu, Q.; Lee, L.Y. Neurophysiology: Neural Control of Airway Smooth Muscle. In Encyclopedia of Respiratory Medicine, Four-Volume Set 2006; Laurent, G.J., Shapiro, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 138–145.
- Cutz, E.; Yeger, H.; Pan, J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2007, 10, 419–435.
- Song, H.; Yao, E.; Lin, C.; Gacayan, R.; Chen, M.H.; Chuang, P.T. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 17531–17536.
- Noguchi, M.; Furukawa, K.T.; Morimoto, M. Pulmonary neuroendocrine cells: Physiology, tissue homeostasis and disease. Dis. Model Mech. 2020, 13, dmm046920.
- Gu, Q.; Lee, l.-y. Neural Control of Airway Smooth Muscle. World J. Anesthesiol. 2020.
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592.
- Toskala, E.; Kennedy, D.W. Asthma risk factors. Int. Forum. Allergy Rhinol. 2015, 5 (Suppl. 1), S11–S16.
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212.
- León, B.; Ballesteros-Tato, A. Modulating Th2 Cell Immunity for the Treatment of Asthma. Front. Immunol. 2021, 12, 637948.
- Barnes, P.J. Th2 cytokines and asthma: An introduction. Respir. Res. 2001, 2, 64.
- Saatian, B.; Rezaee, F.; Desando, S.; Emo, J.; Chapman, T.; Knowlden, S.; Georas, S.N. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 2013, 1, e24333.
- Hellings, P.W.; Steelant, B. Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol. 2020, 145, 1499–1509.
- Burgess, J.K.; Jonker, M.R.; Berg, M.; Ten Hacken, N.T.; Meyer, K.B.; van den Berge, M.; Nawijn, M.C.; Heijink, I.H. Periostin: Contributor to abnormal airway epithelial function in asthma? Eur. Respir. J. 2021, 57, 2001286.
- Schmidt, H.; Braubach, P.; Schilpp, C.; Lochbaum, R.; Neuland, K.; Thompson, K.; Jonigk, D.; Frick, M.; Dietl, P.; Wittekindt, O.H. IL-13 Impairs Tight Junctions in Airway Epithelia. Int. J. Mol. Sci. 2019, 20, 3222.
- Amin, K.; Janson, C.; Bystrom, J. Role of eosinophil granulocytes in allergic airway inflammation endotypes. Scand. J. Immunol. 2016, 84, 75–85.
- Konrádová, V.; Čopová, C.; Suková, B.; Houštěk, J. Ultrastructure of the bronchial epithelium in three children with asthma. Pediatr. Pulmonol. 1985, 1, 182–187.
- Mabalirajan, U.; Dinda, A.K.; Kumar, S.; Roshan, R.; Gupta, P.; Sharma, S.K.; Ghosh, B. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J. Immunol. 2008, 181, 3540–3548.
- Heydeck, D.; Thomas, L.; Schnurr, K.; Trebus, F.; Thierfelder, W.E.; Ihle, J.N.; Kühn, H. Interleukin-4 and -13 induce upregulation of the murine macrophage 12/15-lipoxygenase activity: Evidence for the involvement of transcription factor STAT6. Blood 1998, 92, 2503–2510.
- Rapoport, S.M.; Schewe, T.; Wiesner, R.; Halangk, W.; Ludwig, P.; JanickeHöhne, M.; Tannert, C.; Hiebsch, C.; Klatt, D. The lipoxygenase of reticulocytes. Purification, characterization and biological dynamics of the lipoxygenase; its identity with the 96 respiratory inhibitors of the reticulocyte. Eur. J. Biochem. 1979, 96, 545–561.
- Watson, A.; Doherty, F.J. Calcium promotes membrane association of reticulocyte 15-lipoxygenase. Biochem. J. 1994, 298, 377–383.
- van Leyen, K.; Duvoisin, R.M.; Engelhardt, H.; Wiedmann, M. A function for lipoxygenase in programmed organelle degradation. Nature 1998, 395, 392–395.
- Chu, H.W.; Balzar, S.; Westcott, J.Y.; Trudeau, J.B.; Sun, Y.; Conrad, D.J.; Wenzel, S.E. Expression and activation of 15-lipoxygenase pathway in severe asthma: Relationship to eosinophilic phenotype and collagen deposition. Clin. Exp. Allergy 2002, 32, 1558–1565.
- Chanez, P.; Bonnans, C.; Chavis, C.; Vachier, I. 15-lipoxygenase: A Janus enzyme? Am. J. Respir. Cell Mol. Biol. 2002, 27, 655–658.
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, 1540.
- Heijink, I.H.; Kuchibhotla, V.N.S.; Roffel, M.P.; Maes, T.; Knight, D.A.; Sayers, I.; Nawijn, M.C. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020, 75, 1902–1917.
- Aghapour, M.; Ubags, N.D.; Bruder, D.; Hiemstra, P.S.; Sidhaye, V.; Rezaee, F.; Heijink, I.H. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2022, 31, 210112.
- Loxham, M.; Davies, D.E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 1736–1751.
- Runswick, S.; Mitchell, T.; Davies, P.; Robinson, C.; Garrod, D.R. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007, 12, 834–842.
- Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192.
- Aguilera-Aguirre, L.; Bacsi, A.; Saavedra-Molina, A.; Kurosky, A.; Sur, S.; Boldogh, I. Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol. 2009, 183, 5379–5387.
- Barton, J.L.; Berg, T.; Didon, L.; Nord, M. The pattern recognition receptor Nod1 activates CCAAT/enhancer binding protein beta signalling in lung epithelial cells. Eur. Respir. J. 2007, 30, 214–222.
- Farkas, L.; Stoelcker, B.; Jentsch, N.; Heitzer, S.; Pfeifer, M.; Schulz, C. Muramyldipeptide modulates CXCL-8 release of BEAS-2B cells via NOD2. Scand. J. Immunol. 2008, 68, 315–322.
- Kauffman, H.F. Innate immune responses to environmental allergens. Clin. Rev. Allergy Immunol. 2006, 30, 129–140.
- Kato, A.; Favoreto, S.; Avila, P.C.; Schleimer, R.P. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 2007, 179, 1080–1087.
- Hammad, H.; Lambrecht, B.N. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat. Reviews Immunol. 2008, 8, 193–204.
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr. Opin. Immunol. 2005, 17, 359–365.
- Licona-Limón, P.; Kim, L.K.; Palm, N.W.; Flavell, R.A. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013, 14, 536–542.
- Smithgall, M.D.; Comeau, M.R.; Yoon, B.R.; Kaufman, D.; Armitage, R.; Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008, 20, 1019–1030.
- Gauvreau, G.M.; Bergeron, C.; Boulet, L.P.; Cockcroft, D.W.; Côté, A.; Davis, B.E.; Leigh, R.; Myers, I.; O’Byrne, P.M.; Sehmi, R. Sounding the alarmins-The role of alarmin cytokines in asthma. Allergy 2022, 78, 402–417.
