Halophytes and xerophytes, plants with adequate tolerance to high salinity with strong ability to survive in drought ecosystem, have been recognized for their nutritional and medicinal values owing to their comparatively higher productions of secondary metabolites, primarily the phenolics, and the flavonoids, as compared to the normal vegetation in other climatic regions. Given the consistent increases in desertification around the world, which are associated with increasing salinity, high temperature, and water scarcity, the survival of halophytes due to their secondary metabolic contents has prioritized these plant species, which have now become increasingly important for environmental protection, land reclamation, and food and animal-feed security, with their primary utility in traditional societies as sources of drugs. On the medicinal herbs front, because the fight against cancer is still ongoing, there is an urgent need for development of more efficient, safe, and novel chemotherapeutic agents, than those currently available.
- halophytes
- phenolics
- flavonoids
1. Salt-Tolerant Plants: Halophytes

2. Traditional Uses of Different Halophytes in Cancers and Cancer-Related Symptoms
Medicinal plants have been used as part of complementary and alternative medicines for different cancer types [141][36]. More than 3000 plant species are known for their anticancer activity [142,143][37][38]. Halophytes, widely distributed in the Arabian desert, have been used in traditional medicine for disease prevention and treatment [68,144][29][39]. Certain halophytes have also been used against cancers and in the management of cancer symptoms [145,146,147][40][41][42]. However, published data reporting the ethno-medicinal uses of halophytes in cancer are very limited [72,148][33][43]. However, the Plantago species (Fleaworts, Plantains), Rubia cordifolia (Manjistha, Indian Madder), and Salicornia herbacea (Glasswort) are known medicinal halophytes used against cancers [144][39]. Moreover, the concentrated decoction of Peganum harmala (Harmel), Zizyphus lotus (Wild jujube), Asplenium ceterach L. (Rustyback), and Calendula arvensis L. (Field Marigold) have been reported for tumor management [145,146][40][41]. Seeds of Atriplex halimus (Saltbush) are also used by breast cancer patients in Algeria [147][42]. Several species from the halophytic genus Salsola have been used as a remedy for cancers in Chinese traditional medicine, which includes Salsola tragus (Russian-thistle), Salsola foetida (Zri-che, Ecchi, Ressal, Aghacel), Salsola baryosoma, and Salsola richteri (Kata-kara, Cherkez) [149][44]. The common use of these plants to manage and treat cancers in traditional medicines is an incentive for researchers to further investigate their constituents and examine their effects on various cancer cell lines, as well as their effects in vivo.3. Evaluating Anti-Cancer Activities of Halophytic Plants Extracts
A considerable number of reports are available in which halophytic plant extracts and their fractions have been screened for cytotoxic and antiproliferative effects [150,151,152,153,154][45][46][47][48][49]. Most of these reports evaluated the anticancer activity of plant extracts and fractions that were identified by liquid chromatography–mass spectrometry (LC–MS) and gas chromatography–mass spectrometry (GC–MS) [153,154,155][48][49][50] for the presence of the chemical constituents. Nuclear magnetic resonance (NMR) spectroscopy has also been used for identification and quantification of the chemical constituents and their compositions in the mixture [156][51]. Quantitative spectrophotometric analysis has also been conducted [154,157][49][52]. For instance, n-hexane, dichloromethane, methanol, and water extracts from the xero-halophytic species Reaumuria vermiculata have been phytochemically investigated and evaluated for their anticancer activity together with their anti-inflammatory and antioxidant effects [158][53]. The n-hexane and dichloromethane extracts had the highest cytotoxicity against A-549 lung carcinoma cells (IC50 values of 17 and 23 µg/mL, respectively), while the methanol extract of the plant showed the highest quantities of phenolics and flavonoids [158][53], indicating that the non-polar constituents were responsible for the plant’s anticancer properties. Some studies also demonstrated the anticancer activity of whole-plant extracts. Mohammed, et al. [154][49] reported the anticancer activity of an aqueous alcoholic extract from Pulicaria undulata growing in Saudi Arabia. They found that the plant extract had a potent cytotoxic effect against several cancer cell lines, including MCF-7, K562, and PANC-1, with IC50 values ranging from 519 to 1535 µg/mL. The extract also inhibited normal fibroblast cell growth at IC50 values greater than 4000 µg/mL, indicating the high selectivity index for the cancer cell lines. According to the authors’ conclusions, the accumulation of polyphenols and flavonoids in the plant extract was responsible for the plant’s anticancer effects [154][49]. In addition, the anticancer activities of essential oil constituents of some of the halophytic plants have also been reported. For example, oils obtained from Mentha piperita growing in the Experimental Halophytes Growing Base at the Shandong Academy of Sciences, Jinan, China, exhibited cytotoxic activity in pulmonary carcinoma (SPC-A1, human, lung cancer), K562 (human, chronic myelogenous leukemia), and gastric cancer (SGC-7901, human, first isolated from surgically resected metastatic lymph node) cell lines with IC50 values which ranged from 10.89 to 38.76 µg/mL [159][54]. In vitro and in vivo assays were used to investigate the chemopreventive impacts of halophytes. Six halophytes were also examined in vitro for their stimulation of NAD(P)H: quinone oxidoreductase-1 (NQO-1) in the hepatoma cells (Hepa-1c1c7) murine culture. The results revealed that Ferocactus herrerae, Aptenia cordifolia, Carpobrotus edulis, and Ferocactus glaucescens were the most active chemopreventive plants [160][55]. Tamarix gallica methanolic extract was demonstrated in vivo to have chemopreventive activity against liver cancers, induced by diethylnitrosamine and 2-acetylaminofluorene, which worked through restoring the detoxifying cellular antioxidant enzyme ornithine decarboxylase and DNA synthesis [161][56]. Several investigations on other halophytes have been conducted in similar fashion with the purpose of identifying the plant components and quantifying the presence of key secondary metabolites and their cytotoxic activity (Table 1).| Plant | Location | Active Extract | Main Constituents | Cell Lines/In Vivo Testing, IC50 Values | Proposed Mechanism | References |
|---|---|---|---|---|---|---|
| Anabasis articulata | Saudi Arabia |
Aq. ethanolic extract |
Kaempferol 3-neohesperidosid, 6-gingerol, triterpenes, steroidal saponins, and alkaloids | Panc1 (human pancreatic cancer cell line, derived from ductal cell pancreatic carcinoma), IC50 998.5 | [70][31] | |
| Egypt | Methylene chloride | HePG-2 (human, hepatic carcinoma cell), IC50 6.9; HCT-116, IC50 5.5 | [162][57] | |||
| Arthrocnemum indicum | Tunisia | Shoot aqueous methanol extract | Gallic acid, 3-hydroxy-4′-methoxyflavone, cyanidin, chrysoeriol, quercetin, catechol, syringic acid, luteolin | Shoot extracts inhibited Caco-2 (human, colorectal adenocarcinoma cells) colon cancer cell growth in a dose-dependent manner | Cell cycle blocking at the G2/M phase | [163][58] |
| Arthrocnemum macrostachyum | Egypt | Methanol extract | Phenolic acids and flavonoids | In vivo anticancer effect against Ehrlich solid tumor in mice | Increased tissue necrosis and apoptosis, enhanced DNA fragmentation, upregulated cell cycle regulatory genes (Cdc2 and connexin26), and decreased TNFa levels in tumor tissues | [164][59] |
| Asplenium ceterach | Bulgaria | Aqueous methanol | Phenolic acids and flavonoids | A549 (human, adenocarcinoma, hypotriploid alveolar basal epithelial cells), FL, HeLa (IC50 40.48) | Strong proapoptotic potential against HeLa (human, cervical cancer cell line) |
[165][60] |
| Avicennia marina | Saudi Arabia | Hexane fraction | Betaine and hymecromone | HCT-116, IC50 23.7; HepG2, IC50 44.9; MCF-7, IC50 79.55 | Inhibition of cell cycle in G0/G1 and S phases in HepG2 and MCF-7 | [151][46] |
| Carpobrotus edulis | Portugal | Leaf methanol extract and different fractions. | β-amyrin, uvaol, oleanolic acid, monogalactosyl diacylglycerol, catechin, epicatechin, and procyanidin B5 | L5178 (mouse, T-cell lymphoma cells), and L5178 (mouse, T-cell lymphoma cells) transfected with pHa MDR1/A retrovirus | Inhibition of P-glycoprotein in MDR1-transfected mouse lymphoma cells | [166][61] |
| Chenopodium formosanum | Taiwan | Grain extract | Polyphenols and prebiotic dietary fiber | In vivo colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sulfate sodium in rats | Increase Bax and caspase-9 expressions; reduced TP53 and Bcl-2 expression; decreased expressions of proliferating cell nuclear antigen and cyclooxygenase-2; regulation of apoptosis-related proteins | [167,168][62][63] |
| Mesembryanthemum crystallinum | Korea | Ethanol extracts and its fractions | Phenolics and flavonoids | Inhibition of HCT116 cell growth in dose-dependent manner | Increased G2/M cell population and increased ROS levels in cells | [169][64] |
| Echinophora spinosa | Italy | Essential oils | p-Cymene, β-Phellandrene, β-Phellandrene, myristicin | U937, IC50 14.5–43.4 | Induced apoptosis in U937 cell line (human monocytic cell based) | [170][65] |
| Glaucium flavum | Tunis | Ethyl acetate extract | Isoquinoline alkaloids, kaempferol, caffeic acid, catechin hydrate, syringic acid, chlorogenic acid, isoquercitrin, and trans-hydroxycinnamic acid | MCF-7, IC50 135 | [171][66] | |
| Algeria | CH2Cl2 extract | MDA-MB-435, MDA-MB-231, and Hs578T (IC50 7.9–13.6) as well as in vivo tumor chorioallantoic membrane (CAM) model | Hinders angiogenesis, induction of apoptotic processes, and/or limited neovessel formation inside the tumor | [172][67] | ||
| Iran | Methanol extract and rich alkaloid fraction | HT-29, IC50 22.32 L; Caco-2, IC50 52.38 | [173][68] | |||
| Glehnia littoralis | Korea | Hexane fraction Aqueous methanol fraction |
Furanocoumarin bitter principle and polyacetylene alcohols | HT-29 (77% inhibition at 50 mg/mL extract) | Induced chromatin condensation and nuclear fragmentation, suggesting the presence of apoptotic cells; reduced mRNA expression of Bcl-2, cyclooxygenase (COX-2), and inducible nitric oxide synthase (iNOS) |
[174][69] |
| Limonium densiflorum | Tunisia | CHCl3 extract | Gallic acid, epigallocatechin, quercitrin, myricetin, dihydrokaempferol, isorhamnetin | A-549, IC50 29 µg/mL; DLD-1, IC50 85) | Isorhamnetin induced apoptosis through activation of peroxisome proliferator-activated receptor γ pathway in gastric cancer | [74,175][35][70] |
| Limonium bonduelli | Algeria | n-Butanol extract | Flavonoids (eriodictyol, luteolin, apigenin) and 4-hydroxy-3-methoxy benzoic acid; ethyl acetate extract of L. bonduelli and pure flavonoids, eriodictyol and luteolin | Dose-dependent growth inhibition of HT-29 and HeLa cell-lines | [176][71] | |
| Lotus creticus L | Portugal | Acetone extract (aerial part) Ethanol extract (fruits) |
Steroids, coumarins, tannins, and flavonoids, e.g., catechin, epicatechin, isorhamnetin, quercetin, isorhamnetin-O-hexoside, quercetin-O-hexoside, myricetin-O-hexoside | Extracts had low toxicity RAW 264.7 | [177][72] | |
| Lycium shawii | Saudi Arabia | Aqueous ethanol extract |
Flavonoids, 3-gluco-7-rhamnosyl quercetin, luteolin 7-O-glucoside, kaempferol-3-O-glucoside | MCF7, 194.5 µg/mL; K562, 464.9 µg/mL | Induced apoptosis and cell membrane damage due to necrosis and late apoptosis | [70][31] |
| Malcolmia littorea | Portugal | Polar extracts of flower and roots | Phenolic acids and flavonoids including salicylic acid and luteolin-7-O-glucoside. | HepG2 (viability 38.3%) HEK 293 cells (viability 93.1%) |
[178][73] | |
| Mentha piperita | China | Essential oils | Menthyl acetate, cineol, menthol, pulegone, and caryophyllene oxide | SPC-A1, IC50 10.89; K562, IC50 16.16; and SGC-7901, IC50 38.76. | [159][54] | |
| Pulicaria undulata | Saudi Arabia | Aqueous ethanolic extract | Flavonoids of kaempferol-, luteolin-, and quercetin-based glycosides | MCF-7, 519.2 µg/mL; K562, 1212 µg/mL; PANC-1, 1535 µg/mL |
Cell cycle arrest at the Q1 and Q2 quadrants, and necrosis in late apoptosis | [154][49] |
| Pulicaria crispa | Saudi Arabia | Aqueous ethanolic extract | Sterols, triterpenoids, essential oils, phenolics, and flavonoids | MDA-MB-231, IC50 180 µg/mL | Loss of cancer cell integrity, shrinkage of cytoplasm, and cell detachment | [179][74] |
| Reaumuria vermiculata | Tunisia | Hexane and CH2Cl2 | Myricetin, phenolics, and flavonoids | A-549, IC50 17, (hexane extract), and 23 (dichloromethane extract) | [158][53] | |
| Egypt | Aqueous methanol extract | Huh-7, IC50 2.4; HCT-116, IC50 1.8; MCF-7, IC50 1.3; PC-3, IC50 1.5 | [180][75] | |||
| Salicornia herbacea | Korea | Crude and fine polysaccharide | Polysaccharides and phenolic compounds | HT-29 | Inhibition of cyclin B1 and Cdc2 mRNAG2/M arrest | [181][76] |
| Salvadora persica | Saudi Arabia | Ethanol extracts of fruits | Essential oils, alkaloids, steroids, cetyl dasycarpidan-1-methanol, tetracosamethyl-cyclododecasiloxane, eicosamethyl-cyclodecasiloxane, and 1-monolinoleoylglycerol | MCF7, IC50 17.50; A2780, IC50 8.35; HT29, IC50 5.12 | [182][77] | |
| Salvadora persica L | Egypt | Bark petroleum ether | HepG, IC50 43.6l; MCF-7, IC50 44.3; A549, IC50 19.87 L |
[183][78] | ||
| Suaeda fruticosa | Pakistan | Methanol and CHCl3 extracts | Phenolics, flavonoids, saponins, fatty acids | MCF-7 (63.44% and 45.01% cell viability in methanol and CH2Cl2 at 200 μg/mL), MDA-MB-231 (77.75% and 67.22% cell viability in methanol and dichloromethane at 200 μg/mL), and DU-145 (62.83% and 25.88% cells viability in methanol and dichloromethane at 200 μg/mL) | [184][79] | |
| Tunisia | CH2Cl2 extract | A-549, IC50 49 ± 7; DLD-1, IC50 10 ± 1; Caco-2, IC50 140 ± 13 µg/mL; HT-29, (IC50 12 ± 14 | [157][52] | |||
| Saudi Arabia | Hexane extract | HCT-116, IC50 17.15; MCF-7, IC50 28.1; HepG2, IC50 33.2 | Arrest the cell cycle at the G0-G1 phase | [150][45] | ||
| Tamarix gallica | Tunisia | Methanolic extracts | Phenolic acids and flavonoids | Caco-2, 38% inhibition in cell growth at 100 µg/mL | Decreased DNA synthesis, arrested cell mitosis at G2/M phase; changes in the cell-cycle-associated proteins (cyclin B1, p38, Erk1/2, Chk1, and Chk2) correlated with changes in the cell cycle distribution | [185][80] |
| India | Protects against liver carcinogenesis initiated by diethylnitrosamine and 2-acetylaminofluorene | Restoration of cellular antioxidant enzymes, detoxifying enzymes, ODC activity, and DNA synthesis. | [161][56] | |||
| Zygophyllum album | Tunisia | CH2Cl2 extract | Isorhamnetin-3-O-rutinoside, quinovic acid derivatives, malvidin 3-rhamnoside, quercetin 3-sulfate | A-549, IC50 37; DLD-1, IC50 48 | Downregulation of cyclin B1 and cyclin dependent kinase; upregulation of TP53 and caspase 3 | [155][50] |
| Egypt | HepG2 IC50 27.74 | [186][81] | ||||
| Zygophyllum coccineum | Saudi Arabia | Aqueous ethanolic extract | Phenolics, flavonoids, alkaloids, quinovic acid derivatives. | MCF-7, IC50 3.47; HCT-116, IC50 3.19; HepG2, IC50 2.27 | Inhibition of human topoisomerase-IIβ | [153][48] |
4. Isolated-Purified Anti-Cancer Agents from Different Halophytes
Anticancer activities as anti-proliferative and cytotoxicity of halophytic plant extracts have been widely studied; nonetheless, the anticancer activity of pure chemical compounds isolated, purified, and characterized from halophytes (Figure 2) is less encountered. Flavonoids, the most extensively distributed class of compounds in halophytes, have also been evaluated for their antitumor activity. For example, luteolin, vitexicarpin, and artemetin have been isolated from the halophyte Vitex rotundifolia and tested for their anticancer activity [195][90]. Among the three compounds, vitexicarpin was the most active cytotoxic agent against human gastric adenocarcinoma (AGS) and human colon cancer HT-29 cell lines, with IC50 values of 6.9 and 22.8 µM, respectively. Vitexicarpin also induced apoptosis by upregulating the expression of TP53 and p21 and downregulating the expression of Bcl-2 at a concentration of 25 µM [195][90]. Catechin, epicatechin, and procyanidin B5 were isolated from Carpobrotus edulis and shown to possess cytotoxic activity against MDR1-transfected mouse lymphoma (L5178) cells, with IC50 values equal to 12, 6, and 13 mg/L of the pure compounds, respectively [166][61]. In addition, three triterpenoids and a monogalactosyl diacylglycerol compound isolated from the same plant demonstrated antiproliferative potential in MDR1-transfected L5178 cells. In addition, uvaol (triterpene) exhibited the highest relative fluorescence factor (FAR) value and considerable inhibition of P-glycoprotein [166][61]. Halophytes are also a rich source of phenolic acids since they abundantly produce phenolics as a defensive mechanism against the oxidative stress of salinity. Therefore, phenolics have been represented in all reported phytochemical analyses of halophytes. In addition, some reports have investigated the isolation and anticancer effects of halophyte phenolics. For example, several phenolics have been isolated from Tamarix nilotica, including stamarixinin A (ellagitannin), gallic acid, methyl gallate, and 3,4-di-O-methylgallic acid, and these products have been examined for their antiproliferative activity against lung adenocarcinoma cell lines A549 [196][91]. Among these four compounds, gallic acid exhibited the highest cytotoxic effect, with an IC50 value of 10.5 µg/mL. Other classes of natural products have also been identified from halophytes and found to exhibit anticancer activity. For instance, bitter principles of the furanocoumarin-type, i.e., bergapten, isopimpinellin, xanthotoxin, and imperatorin, as well as polyacetylene alcohols, i.e., panaxydiol, falcaindiol, and falcarinol, were isolated from Glehnia littoralis, a halophytic species, and were shown to have dose-dependent antiproliferative effects against HT-29 cell lines. Among all of the isolated compounds, falcaindiol was the most active cytotoxic agent against HT-29, with an IC50 of 35 µM [174][69]. The pure alkaloid bocconoline, isolated from Glaucium flavum, demonstrated strong cytotoxic effects against MDA-MB-231 cell lines, with an IC50 of 7.8 µM [197][92]. Juncunol (7-vinyl-9,10-dihydro-1,6-dimethylphenanthren-2-ol) was isolated from Juncus acutus ether extract, and exhibited potential cytotoxic activity against HepG2, HeLa, and MDA-MB-468, with IC50 values ranging from 13 to 20 µM together with a highly selective index compared to its effect on the normal cell lines mTEC and S17 [198][93]. Finally, Salicornia herbacea polysaccharide inhibited cell growth and induced apoptosis in HT-29 cell lines [181][76]. The mechanisms by which these polysaccharides exhibit their anticancer activity has been attributed to their effectiveness in inducing G2/M cell cycle arrest at a dose of 4 mg/mL and the inhibition of cyclin B1 and Cdc2 mRNA, which leads to inhibition of HT-29 cell-line proliferation [181][76].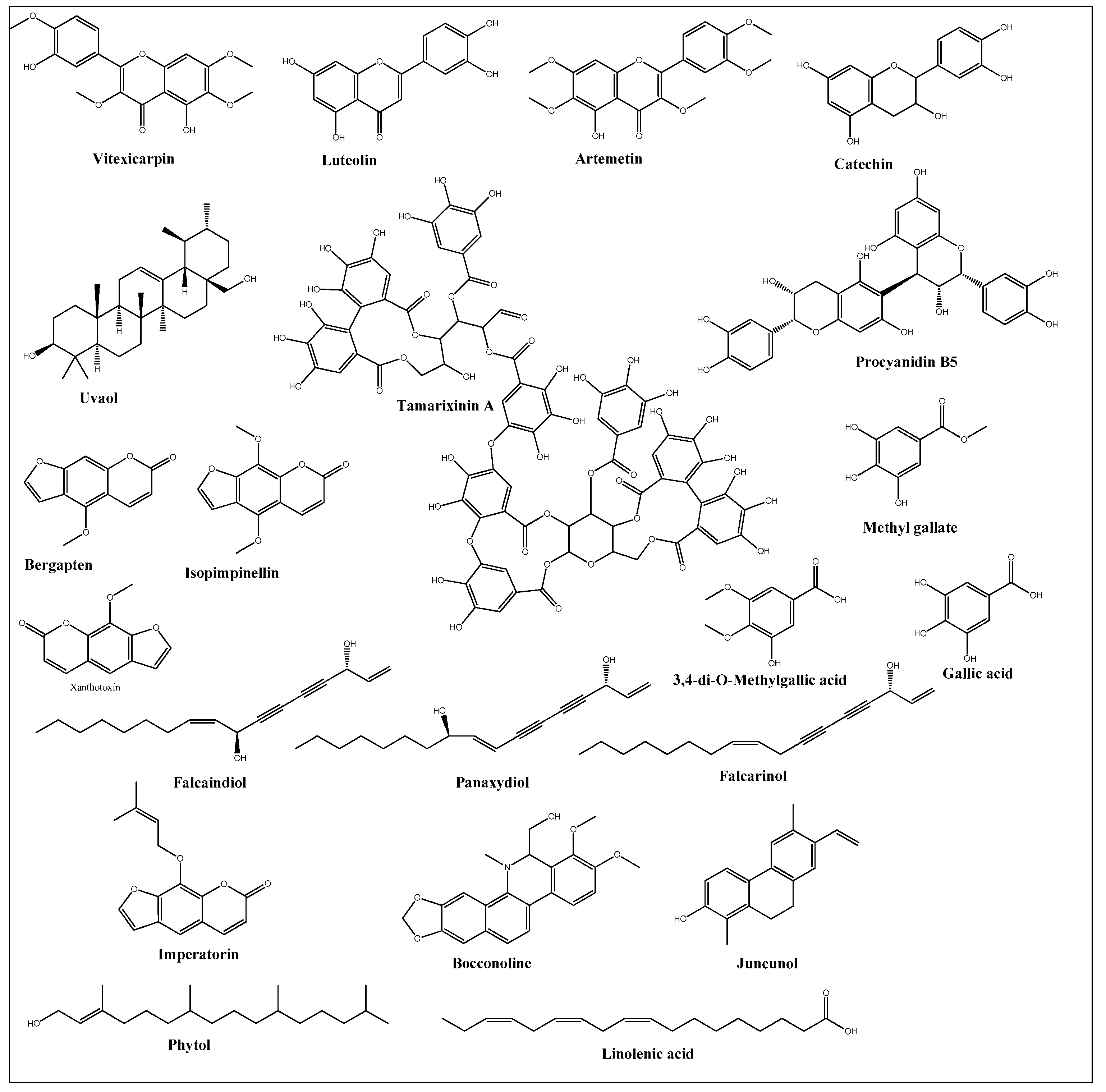
References
- Shabala, S.; Mackay, A. Ion Transport in Halophytes. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 57, pp. 151–199. ISBN 0065-2296.
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The Mechanism of Salt Tolerance in Halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121.
- Manousaki, E.; Kalogerakis, N. Halophytes—An Emerging Trend in Phytoremediation. Int. J. Phytoremediation 2011, 13, 959–969.
- O’Leary, J.W.; Glenn, E.P. Global Distribution and Potential for Halophytes. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Springer: Berlin/Heidelberg, Germany, 1994; pp. 7–17.
- Al-Azzawi, M.J.; Flowers, T.J. Distribution and Potential Uses of Halophytes within the Gulf Cooperation Council States. Agronomy 2022, 12, 1030.
- Batanouny, K.H. Halophytes and Halophytic Plant Communities in the Arab Region. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Springer: Berlin/Heidelberg, Germany, 1994; pp. 139–163.
- Khan, M.A.; Qaiser, M. Halophytes of Pakistan: Characteristics, Distribution and Potential Economic Usages. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2006; pp. 129–153.
- Waisel, Y. Biology of Halophytes; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0323151582.
- Flowers, T.J.; Muscolo, A. Introduction to the Special Issue: Halophytes in a Changing World. AoB Plants 2015, 7, plv020.
- Faustino, M.V.; Faustino, M.A.F.; Pinto, D.C.G.A. Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities. Int. J. Mol. Sci. 2019, 20, 1067.
- Xu, C.; Tang, X.; Shao, H.; Wang, H. Salinity Tolerance Mechanism of Economic Halophytes from Physiological to Molecular Hierarchy for Improving Food Quality. Curr. Genom. 2016, 17, 207–214.
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative Stress Responses of Some Endemic Plants to High Altitudes by Intensifying Antioxidants and Secondary Metabolites Content. Plants 2020, 9, 869.
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A.K. Antioxidative Response Mechanisms in Halophytes: Their Role in Stress Defence. J. Genet. 2006, 85, 237.
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological Adaptations of Two Halophytes to Salt Stress: Photosynthesis, PS II Photochemistry and Anti-Oxidant Feedback–Implications for Resilience in Climate Change. Plant Physiol. Biochem. 2013, 67, 178–188.
- Pang, C.-H.; Wang, B.-S. Oxidative Stress and Salt Tolerance in Plants. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2008; pp. 231–245.
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile Maritima. Plant Physiol. Biochem. 2007, 45, 244–249.
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of Biological, Environmental and Technical Factors on Phenolic Content and Antioxidant Activities of Tunisian Halophytes. Comptes Rendus Biol. 2008, 331, 865–873.
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive Oxygen Species Regulation and Antioxidant Defence in Halophytes. Funct. Plant Biol. 2013, 40, 832–847.
- Abideen, Z.; Qasim, M.; Rasheed, A.; Adnan, M.Y.; Gul, B.; Khan, M.A. Antioxidant Activity and Polyphenolic Content of Phragmites Karka under Saline Conditions. Pak. J. Bot. 2015, 47, 813–818.
- Boughalleb, F.; Denden, M. Physiological and Biochemical Changes of Two Halophytes, Nitraria retusa (Forssk.) and Atriplex halimus (L.) under Increasing Salinity. Agric. J. 2011, 6, 327–339.
- Ben Taârit, M.; Msaada, K.; Hosni, K.; Marzouk, B. Physiological Changes, Phenolic Content and Antioxidant Activity of Salvia officinalis L. Grown under Saline Conditions. J. Sci. Food Agric. 2012, 92, 1614–1619.
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326.
- Bayoumi, M.T.; El Shaer, H.M. Impact of Halophytes on Animal Health and Nutrition. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Springer: Berlin/Heidelberg, Germany, 1994; pp. 267–272.
- Cybulska, I.; Brudecki, G.; Alassali, A.; Thomsen, M.; Brown, J.J. Phytochemical Composition of Some Common Coastal Halophytes of the United Arab Emirates. Emir. J. Food Agric. 2014, 26, 1046–1057.
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762.
- Yadav, S.; Elansary, H.O.; Mattar, M.A.; M Elhindi, K.; A Alotaibi, M.; Mishra, A. Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions. Agronomy 2021, 11, 131.
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C. Atriplex Mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis. Molecules 2018, 23, 1962.
- Qasim, M.; Gulzar, S.; Shinwari, Z.K.; Aziz, I.; Khan, M.A. Traditional Ethnobotanical Uses of Halophytes from Hub, Balochistan. Pak. J. Bot 2010, 42, 1543–1551.
- Qasim, M.; Gulzar, S.; Khan, M.A. Halophytes as Medicinal Plants. In Urbanisation, Land Use, Land Degradation and Environment; Springer: Berlin/Heidelberg, Germany, 2011.
- Al-Omar, M.S.; Mohammed, H.A.; Mohammed, S.A.A.; Abd-Elmoniem, E.; Kandil, Y.I.; Eldeeb, H.M.; Chigurupati, S.; Sulaiman, G.M.; Al-Khurayyif, H.K.; Almansour, B.S. Anti-Microbial, Anti-Oxidant, and α-Amylase Inhibitory Activity of Traditionally-Used Medicinal Herbs: A Comparative Analyses of Pharmacology, and Phytoconstituents of Regional Halophytic Plants’ Diaspora. Molecules 2020, 25, 5457.
- Mohammed, H.A.; Ali, H.M.; Qureshi, K.A.; Alsharidah, M.; Kandil, Y.I.; Said, R.; Mohammed, S.A.A.; Al-Omar, M.S.; Al Rugaie, O.; Abdellatif, A.A.H. Comparative Phytochemical Profile and Biological Activity of Four Major Medicinal Halophytes from Qassim Flora. Plants 2021, 10, 2208.
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140.
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Ansari, R.; Gul, B.; Khan, M.A. Traditional Ethnobotanical Uses of Medicinal Plants from Coastal Areas. J. Coast Life Med. 2014, 2, 22–30.
- Medini, F.; Ksouri, R. Antimicrobial Capacities of the Medicinal Halophyte Plants. In Natural Antimicrobial Agents; Springer: Berlin/Heidelberg, Germany, 2018; pp. 271–288.
- Medini, F.; Legault, J.; Pichette, A.; Abdelly, C.; Ksouri, R. Antiviral Efficacy of Limonium Densiflorum against HSV-1 and Influenza Viruses. South Afr. J. Bot. 2014, 92, 65–72.
- Jacobo-Herrera, N.J.; Jacobo-Herrera, F.E.; Zentella-Dehesa, A.; Andrade-Cetto, A.; Heinrich, M.; Pérez-Plasencia, C. Medicinal Plants Used in Mexican Traditional Medicine for the Treatment of Colorectal Cancer. J. Ethnopharmacol. 2016, 179, 391–402.
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants Used against Cancer–an Extension of the Work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377.
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican Medicinal Plants Used for Cancer Treatment: Pharmacological, Phytochemical and Ethnobotanical Studies. J. Ethnopharmacol. 2011, 133, 945–972.
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Kumar, A.; Chand, G. Halophytes: The Plants of Therapeutic Medicine. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 271–287.
- Hammiche, V.; Maiza, K. Traditional Medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367.
- Bozyel, M.E.; Bozyel, E.M.; Canli, K.; Altuner, E.M. Anticancer Uses of Medicinal Plants in Turkish Traditional Medicine. J. Agric. Nat. 2019, 22, 465–484.
- Benarba, B. Use of Medicinal Plants by Breast Cancer Patients in Algeria. EXCLI J. 2015, 14, 1164.
- Stevanovic, Z.D.; Stankovic, M.S.; Stankovic, J.; Janackovic, P.; Stankovic, M. Use of Halophytes as Medicinal Plants: Phytochemical Diversity and Biological Activity. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford, UK, 2019; p. 343.
- Seo, J.H.; Jin, M.H.; Chang, Y.H. Anti-Inflammatory Effect of Salsola Komarovii Extract with Dissociated Glucocorticoid Activity. BMC Complement. Med. Ther. 2020, 20, 176.
- Saleh, K.A.; Albinhassan, T.H.; Al-Ghazzawi, A.M.; Mohaya, A.; Shati, A.A.; Ayoub, H.J.; Abdallah, Q.M. Anticancer Property of Hexane Extract of Suaeda Fruticose Plant Leaves against Different Cancer Cell Lines. Trop. J. Pharm. Res. 2020, 19, 129–136.
- Albinhassan, T.H.; Saleh, K.A.; Barhoumi, Z.; Alshehri, M.A.; Al-Ghazzawi, A.M. Anticancer, Anti-Proliferative Activity of Avicennia Marina Plant Extracts. J. Cancer Res. Ther. 2021, 17, 879.
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Alhowail, A.H.; Eldeeb, H.M.; Sajid, M.S.M.; Abd-Elmoniem, E.M.; Alghulayqeh, O.A.; Kandil, Y.I.; Khan, R.A. Phytochemical Analysis, Pharmacological and Safety Evaluations of Halophytic Plant, Salsola cyclophylla. Molecules 2021, 26, 2384.
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A. Phytochemical Profiling, In Vitro and In Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent Fra. Molecules 2021, 26, 577.
- Mohammed, H.A.; Al-Omar, M.S.; Khan, R.A.; Mohammed, S.A.A.; Qureshi, K.A.; Abbas, M.M.; Al Rugaie, O.; Abd-Elmoniem, E.; Ahmad, A.M.; Kandil, Y.I. Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert. Plants 2021, 10, 1811.
- Ksouri, W.M.; Medini, F.; Mkadmini, K.; Legault, J.; Magné, C.; Abdelly, C.; Ksouri, R. LC–ESI-TOF–MS Identification of Bioactive Secondary Metabolites Involved in the Antioxidant, Anti-Inflammatory and Anticancer Activities of the Edible Halophyte Zygophyllum album Desf. Food Chem. 2013, 139, 1073–1080.
- Dhahri, M.; Sioud, S.; Dridi, R.; Hassine, M.; Boughattas, N.A.; Almulhim, F.; Al Talla, Z.; Jaremko, M.; Emwas, A.-H.M. Extraction, Characterization, and Anticoagulant Activity of a Sulfated Polysaccharide from Bursatella Leachii Viscera. ACS Omega 2020, 5, 14786–14795.
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic Content, Antioxidant, Anti-Inflammatory and Anticancer Activities of the Edible Halophyte Suaeda Fruticosa Forssk. Food Chem. 2012, 132, 943–947.
- Karker, M.; Falleh, H.; Msaada, K.; Smaoui, A.; Abdelly, C.; Legault, J.; Ksouri, R. Antioxidant, Anti-Inflammatory and Anticancer Activities of the Medicinal Halophyte Reaumuria Vermiculata. EXCLI J. 2016, 15, 297.
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha Piperita Grown in China. PLoS ONE 2014, 9, e114767.
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS—Bioassay Guided Approach. J. Chromatogr. Sci. 2021, 59, 618–626.
- Sehrawat, A.; Sultana, S. Evaluation of Possible Mechanisms of Protective Role of Tamarix gallica against DEN Initiated and 2-AAF Promoted Hepatocarcinogenesis in Male Wistar Rats. Life Sci. 2006, 79, 1456–1465.
- Gamal, G.; Abo-El-Seoud, K.A.; Attia, G. Triterpenoids from the Aerial Parts of Anabasis articulata (Forssk) Moq: Gastroprotective Effect in Vivo with in Silico Studies, Cytotoxic and Antimicrobial Activities. Nat. Prod. Res. 2021, 36, 4076–4084.
- Boulaaba, M.; Mkadmini, K.; Tsolmon, S.; Han, J.; Smaoui, A.; Kawada, K.; Ksouri, R.; Isoda, H.; Abdelly, C. In Vitro Antiproliferative Effect of Arthrocnemum indicum Extracts on Caco-2 Cancer Cells through Cell Cycle Control and Related Phenol LC-TOF-MS Identification. Evid.-Based Complement. Altern. Med. 2013, 2013, 529375.
- Sharawi, Z.W. Therapeutic Effect of Arthrocnemum machrostachyum Methanolic Extract on Ehrlich Solid Tumor in Mice. BMC Complement. Med. Ther. 2020, 20, 153.
- Petkov, V.; Batsalova, T.; Stoyanov, P.; Mladenova, T.; Kolchakova, D.; Argirova, M.; Raycheva, T.; Dzhambazov, B. Selective Anticancer Properties, Proapoptotic and Antibacterial Potential of Three Asplenium Species. Plants 2021, 10, 1053.
- Martins, A.; Vasas, A.; Schelz, Z.S.; Viveiros, M.; Molnar, J.; Hohmann, J.; Amaral, L. Constituents of Carpobrotus edulis Inhibit P-Glycoprotein of MDR1-Transfected Mouse Lymphoma Cells. Anticancer Res. 2010, 30, 829–835.
- Lee, C.-W.; Chen, H.-J.; Xie, G.-R.; Shih, C.-K. Djulis (Chenopodium formosanum) Prevents Colon Carcinogenesis via Regulating Antioxidative and Apoptotic Pathways in Rats. Nutrients 2019, 11, 2168.
- Lee, C.-W.; Chen, H.-J.; Chien, Y.-H.; Hsia, S.-M.; Chen, J.-H.; Shih, C.-K. Synbiotic Combination of Djulis (Chenopodium formosanum) and Lactobacillus acidophilus Inhibits Colon Carcinogenesis in Rats. Nutrients 2020, 12, 103.
- Seo, J.A.; Ju, J. Antioxidant and Growth Inhibitory Activities of Mesembryanthemum crystallinum L. in HCT116 Human Colon Cancer Cells. J. Nutr. Health 2019, 52, 157–167.
- Fraternale, D.; Ricci, D.; Calcabrini, C.; Guescini, M.; Martinelli, C.; Sestili, P. Cytotoxic Activity of Essential Oils of Aerial Parts and Ripe Fruits of Echinophora spinosa (Apiaceae). Nat. Prod. Commun. 2013, 8, 1645–1649.
- Boulaaba, M.; Kalai, F.Z.; Dakhlaoui, S.; Ezzine, Y.; Selmi, S.; Bourgou, S.; Smaoui, A.; Isoda, H.; Ksouri, R. Antioxidant, Antiproliferative and Anti-Inflammatory Effects of Glaucium flavum Fractions Enriched in Phenolic Compounds. Med. Chem. Res. 2019, 28, 1995–2001.
- Bournine, L.; Bensalem, S.; Peixoto, P.; Gonzalez, A.; Maiza-Benabdesselam, F.; Bedjou, F.; Wauters, J.-N.; Tits, M.; Frederich, M.; Castronovo, V. Revealing the Anti-Tumoral Effect of Algerian Glaucium flavum Roots against Human Cancer Cells. Phytomedicine 2013, 20, 1211–1218.
- Haji, A.F.; Ostad, S.N.; Khanavi, M.; Haji, A.A.; Farahanikia, B.; Salarytabar, A. Cytotoxicity of Two Species of Glaucium from Iran. J. Med. Plants 2013, 12, 85–92.
- Um, Y.R.; Kong, C.-S.; Im Lee, J.; Kim, Y.A.; Nam, T.J.; Seo, Y. Evaluation of Chemical Constituents from Glehnia littoralis for Antiproliferative Activity against HT-29 Human Colon Cancer Cells. Process Biochem. 2010, 45, 114–119.
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Abdelly, C.; Legault, J.; Ksouri, R. Phytochemical Analysis, Antioxidant, Anti-Inflammatory, and Anticancer Activities of the Halophyte Limonium densiflorum Extracts on Human Cell Lines and Murine Macrophages. South Afr. J. Bot. 2015, 99, 158–164.
- Amrani, A.; Lahneche, A.M.; Benaissa, O.; Boubekri, N.; Demirtaş, I.; Benayache, F.; Benayache, S.; Zama, D. In Vitro Antiproliferative and Inhibition of Oxidative DNA Damage Activities of N-Butanol Extract of Limonium bonduelli from Algeria. Braz. Arch. Biol. Technol. 2019, 62.
- Placines, C.; Castaneda-Loaiza, V.; Rodrigues, M.J.; Pereira, C.G.; da Silva, J.P.; Zengin, G.; Custódio, L. In Vitro Enzyme Inhibitory and Antioxidant Properties, Cytotoxicity, and LC-DAD-ESI-MS/MS Profile of Extracts from the Halophyte Lotus creticus L. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e101125.
- Castañeda-Loaiza, V.; Placines, C.; Rodrigues, M.J.; Pereira, C.G.; Zengin, G.; Neng, N.R.; Nogueira, J.M.F.; Custódio, L. In Vitro Enzyme Inhibitory and Anti-Oxidant Properties, Cytotoxicity and Chemical Composition of the Halophyte Malcolmia littorea (L.) R. Br. (Brassicaceae). Nat. Prod. Res. 2020, 35, 4753–4756.
- Barnawi, I.O.; Ali, I. Anticancer Potential of Pulicaria Crispa Extract on Human Breast Cancer MDA-MB-231 Cells. Lett. Drug Des. Discov. 2019, 16, 1354–1359.
- Nawwar, M.A.; Ayoub, N.A.; El-Rai, M.A.; Bassyouny, F.; Mostafa, E.S.; Al-Abd, A.M.; Harms, M.; Wende, K.; Lindequist, U.; Linscheid, M.W. Cytotoxic Ellagitannins from Reaumuria vermiculata. Fitoterapia 2012, 83, 1256–1266.
- Ryu, D.-S.; Kim, S.-H.; Lee, D.-S. Anti-Proliferative Effect of Polysaccharides from Salicornia Herbacea on Induction of G2/M Arrest and Apoptosis in Human Colon Cancer Cells. J. Microbiol. Biotechnol. 2009, 19, 1482–1489.
- Al Bratty, M.; Makeen, H.A.; Alhazmi, H.A.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W.; Khalid, A. Phytochemical, Cytotoxic, and Antimicrobial Evaluation of the Fruits of Miswak Plant, Salvadora persica L. J. Chem. 2020, 2020, 4521951.
- Ibrahim, A.Y.; El-Gengaihi, S.E.; Motawe, H.M. Phytochemical and Cytotoxicity Investigations of Salvadora persica Bark Extracts. JASMR 2011, 6, 127–133.
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M. A Comprehensive Phytochemical, Biological, Toxicological and Molecular Docking Evaluation of Suaeda fruticosa (L.) Forssk.: An Edible Halophyte Medicinal Plant. Food Chem. Toxicol. 2021, 154, 112348.
- Boulaaba, M.; Tsolmon, S.; Ksouri, R.; Han, J.; Kawada, K.; Smaoui, A.; Abdelly, C.; Isoda, H. Anticancer Effect of Tamarix gallica Extracts on Human Colon Cancer Cells Involves Erk1/2 and P38 Action on G 2/M Cell Cycle Arrest. Cytotechnology 2013, 65, 927–936.
- El-Attar, M.M.; Abdel-Tawab, F.M.; Awad, A.A.; Ahmad, E.S.; Kamel, H.A.; Hassan, A.I. Anti-Cancer Effects of Zygophyllum album and Suaeda palaestina Extracts on Human Liver Cancer Cell Lines. Egypt. J. Genet. Cytol. 2019, 48, 77–90.
- Nipun, T.S.; Khatib, A.; Ibrahim, Z.; Ahmed, Q.U.; Redzwan, I.E.; Saiman, M.Z.; Supandi, F.; Primaharinastiti, R.; El-Seedi, H.R. Characterization of α-Glucosidase Inhibitors from Psychotria malayana Jack Leaves Extract Using LC-MS-Based Multivariate Data Analysis and In-Silico Molecular Docking. Molecules 2020, 25, 5885.
- Noumi, E.; Snoussi, M.; Anouar, E.H.; Alreshidi, M.; Veettil, V.N.; Elkahoui, S.; Adnan, M.; Patel, M.; Kadri, A.; Aouadi, K. HR-LCMS-Based Metabolite Profiling, Antioxidant, and Anticancer Properties of Teucrium polium L. Methanolic Extract: Computational and In Vitro Study. Antioxidants 2020, 9, 1089.
- Safitri, A.; Putri, A.S.; Octavianty, T.D.; Sari, D.R.T. Metabolomic Profiles of Curcuma Longa L and Cosmos Caudatus Extracts and Their In-Silico Anti-Cancer Activity. J. Phys. Conf. Ser. 2020, 1665, 12022.
- Sahu, R.; Kar, R.K.; Sunita, P.; Bose, P.; Kumari, P.; Bharti, S.; Srivastava, S.; Pattanayak, S.P. LC-MS Characterized Methanolic Extract of Zanthoxylum armatum Possess Anti-Breast Cancer Activity through Nrf2-Keap1 Pathway: An In-Silico, In-Vitro and In-Vivo Evaluation. J. Ethnopharmacol. 2021, 269, 113758.
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In Vitro and In Silico Perspectives on Biological and Phytochemical Profile of Three Halophyte Species—A Source of Innovative Phytopharmaceuticals from Nature. Phytomedicine 2018, 38, 35–44.
- Mohammed, H.A.; Almahmoud, S.A.; Arfeen, M.; Srivastava, A.; El-Readi, M.Z.; Ragab, E.A.; Shehata, S.M.; Mohammed, S.A.A.; Mostafa, E.M.; El-khawaga, H.A.; et al. Phytochemical Profiling, Molecular Docking, and In Vitro Anti-Hepatocellular Carcinoid Bioactivity of Suaeda Vermiculata Extracts. Arab. J. Chem. 2022, 15, 103950.
- Farooq Khan, M.; Nasr, F.A.; Baabbad, A.A.; Alqahtani, A.S.; Wadaan, M.A.M. Investigating the Anticancer Activity and Characterization of Bioactive Constituents of Moricandia sinaica (Boiss.) Boiss through In Vitro and In Silico Approaches in Triple-Negative Breast Cancer Cell Line. Appl. Sci. 2021, 11, 1244.
- Mohammed, H.; Al-Mahmoud, S.; Arfeen, M.; Srivastava, A.; Al-Raidy, M.Z.; Ragab, E.A.; Shehata, S.M.; Mohammed, S.A.A.; Mostafa, E.M.; El-Khawaga, H.A. LC-MS Profiled Chemical Constituents, Molecular Modeling, and In Vitro Bioactivity Evaluations of Suaeda vermiculata Extracts as Anti-Hepatocellular Carcinoma Preparation: Assessment of the Constituents’ Role, and Receptor Docking Feasibility Based Activity Projections. Arab. J. Chem. 2022, 15, 103950.
- Kim, Y.A.; Kim, H.; Seo, Y. Antiproliferative Effect of Flavonoids from the Halophyte Vitex rotundifolia on Human Cancer Cells. Nat. Prod. Commun. 2013, 8, 1405–1408.
- Orabi, M.A.A.; Zidan, S.A.H.; Attia, G.H.; Alyami, H.S.; Matsunami, K.; Hatano, T. Ellagitannins and Simple Phenolics from the Halophytic Plant Tamarix nilotica. Nat. Prod. Res. 2020, 36, 177–185.
- Bournine, L.; Bensalem, S.; Wauters, J.-N.; Iguer-Ouada, M.; Maiza-Benabdesselam, F.; Bedjou, F.; Castronovo, V.; Bellahcène, A.; Tits, M.; Frédérich, M. Identification and Quantification of the Main Active Anticancer Alkaloids from the Root of Glaucium flavum. Int. J. Mol. Sci. 2013, 14, 23533–23544.
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules. Mar. Drugs 2014, 12, 2228–2244.
