Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Abidemi Oluranti Ojo.
The lactic acid annual global market in 2020 was valued at 1.1 billion US dollars and is expected to have a compound annual growth rate (CAGR) of 8% from 2021 to 2028. Lactic acid usage in end-use industries such as pharmaceuticals, biomedicals, foods, and beverages drives demand over the forecast period. Lactic acid is produced through chemical or microbial fermentative processes.
- lactic acid
- lignocellulose
- pretreatments
- fermentation
1. Chemical Synthesis of Lactic Acid Production
Lactic acid is produced by various chemical reactions, including (i) hydrolysis of lactic acid derivatives, e.g., esters or nitriles, (ii) hydrolysis of the other two substituted propionic acids, (iii) decarboxylation of some derivatives of 2-methylmalonic acid, (iv) reduction, (v) oxidation and (vi) rearrangement and disproportion [1]. However, only lactic acid synthesis from its derivatives has been commercialized. Though the chemical synthesis of lactic acid is not economically feasible [2], several studies have reported the chemical synthesis of lactic acid using different carbon sources. For example, lactic acid can be chemically synthesized from a petrochemical source. The reaction steps in lactic acid production using petrochemical sources include the oxidation of ethene in the presence of palladium (II) chloride to form acetaldehyde (Figure 1A). The acetaldehyde in the liquid phase under high pressure with hydrogen cyanide in the presence of a base is converted into lactonitrile. Lactonitrile is recovered and purified, and sulphuric acid is used to hydrolyze lactonitrile to form a racemic mixture of L- and D-lactic acid [3]. The reactions in the synthesis of lactic acid or its lactate involve the addition of glycerol, water, and a catalyst (sodium or potassium hydroxide) into a batch reactor equipped with a magnetic stirrer set at 800 rpm and temperature (240–247 °C; 260–270 °C) (Figure 1B) [4].
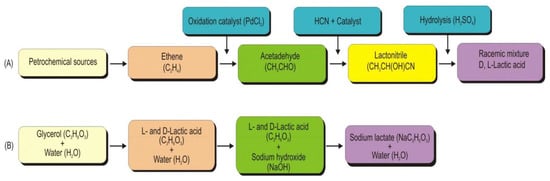
Figure 1. Block diagram of chemical synthesis of lactic acid using sources and conditions. (A): Synthesis of lactic acid using petrochemical source and (B): Alkaline hydrothermal conversion of glycerol to lactic acid and its lactate (Adapted from Yankov (2022) [3]).
Alkaline hydrothermal conversion of glycerol yielded chemical synthesis of a racemic mixture of L- and D-lactic acid [4,5][4][5]. Zhou et al. (2010) [6] have shown that lactic acid could also be synthesized from mannitol (C6polyol) through alkaline hydrothermal conditions; however, the lactic acid yield from mannitol was lower than the yield obtained from glucose and glycerine. Other routes of chemical synthesis of lactic acid include oxidation of propylene glycol at a low temperature [7], conversion of propene into α-nitropropionic acid using nitric acid in the presence of oxygen, and α-nitropropionic acid is then hydrolyzed into lactic acid [1].
2. Fermentative Production of Lactic Acid
Over 90% of lactic acid is produced through microbial fermentation of carbon sources [4]. In fermentation, fermentable sugars in nutrient supplements are converted to lactic acid by capable microorganisms under favorable conditions [8]. The selection of favorable conditions for fermentative lactic acid production is vital. Factors such as temperature, pH, nutrients, substrate concentration, and end-product concentration, among others, were reported to impact fermentation [9]. The temperature and pH are important conditions in lactic acid fermentation as they are associated with cellular metabolism, which affects the growth of microorganisms, substrate consumption, and lactic acid production [9].
Consequently, the optimum temperature is selected and maintained. The selected optimum pH is maintained by adding a strong base such as calcium hydroxide, sodium hydroxide, or potassium hydroxide during fermentation due to lactic acid production that lowers the pH [10]. Nutrients can influence lactic acid production since microorganisms such as lactic acid-producing bacteria require complex nutrients. The carbon source available as sugars is vital for lactic acid-producing bacteria (LAB) reproduction. Minerals, vitamins, and nitrogen available as inorganic compounds are essential for microbial growth, maintenance, and production of lactic acid [9]. High substrate concentration due to the overloading of carbon sources or the inability of the selected microorganisms to utilize substrates can lead to an inhibition [8,9][8][9]. The end-product of the fermentation process could give inhibitory effects because of the accumulation of lactic acid in the system, thereby causing a decrease in cell growth, extended fermentation period, and reduced lactic acid productivity [8,11][8][11].
3. Industrial/Commercial Fermentative Lactic Acid Production and Purification
Commercial/industrial production of lactic acid involves fermentation of the fermentable sugars and the purification of the fermentation broth to obtain pure lactic acid. In general, fermentative production of lactic acid can utilize standard fermentation technology [12]. Presently, lactic acid is commercially produced from starch fermentation. However, the generation of lactic acid by fermentation of renewable agricultural feedstock resources such as lignocellulosic materials, food wastes, dairy wastes, and beverage industry wastes that contain fermentable sugars reduces the production cost, renders waste into a resource and reduces challenges in the environmental disposal of the wastes [13,14][13][14]. Little information is made available on the extensive use of lignocellulosic materials for the commercial/industrial production of lactic acid. However, Agblevor and Evans (2004) [15] presented an industrial method for producing lactic acid from agricultural livestock, specifically lignocellulosic materials such as soybean hulls. This industrial method includes size reduction by mechanical or steam explosion pretreatment, followed by hydrolysis, which may be either enzymatic or acid hydrolysis, and fermentation. In the industrial production of lactic acid, hydrolysis, and fermentation can be conducted simultaneously or separately [15]. For instance, in the industrial production of lactic acid, enzyme hydrolysis of the pretreated material and microbial fermentation occur simultaneously in the same vessel (SmSF). Or the pretreated material is first subjected to enzymatic hydrolysis/saccharification, followed by microbial fermentation of the hydrolysate (SHF) [15,16][15][16]. It should be noted that the microorganism selected for fermentation allows stereospecific lactic acid to be produced as desired. For instance, reports have shown that homofermentative LAB such as Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Lactobacillus helveticus, and fungi species from the genus Rhizopus, with their amylolytic enzyme activity, produce L-lactic acid, which can be used as a precursor for polylactic acid (PLA) production [17-20][17][18][19][20]. Different methods have been used in the commercial production of lactic acid; the most common methods include classical calcium lactate and ammonium lactate processes [1]. Generally, the mode used during fermentation varies from batch, fed-batch, repeated batch to continuous fermentation, etc. [1,2][1][2]. Continuous fermentation mode offers high lactic acid productivity, while fed-batch fermentation mode gives high lactic acid yield. However, batch modes are commonly used in the industry for lactic acid production [12].3.1. Classical Calcium Lactate Process for Lactic Acid Production and Purification
In the classical calcium lactate process of lactic acid production, a non-corrosive reactor is usually used for fermentation as the corrosion of the vessel can lead to contamination of the fermentation fluid by soluble heavy metals. In classical lactic acid manufacturing, fermentation is done in batch mode. Culture and medium containing a fermentable sugar, e.g., glucose with a concentration between 120 and 180 g·L−1, and complex nitrogen sources (e.g., the mixture of inorganic nitrogen such as ammonium phosphate and ammonia phosphate) with complex organic materials such as yeast extracts, peptone, etc. that yields between 1 and 10 g·L−1 are added to the reactor [1]. Fermentation is conducted in reactor volumes of more than 100 m3 and at a temperature higher than 40 °C, depending on the microorganism used. For instance, if L. delbrueckii is used, the temperature is set up to 50 °C; possible contamination could be avoided at this temperature [1]. Agitation is done during fermentation, and calcium carbonate is added in increments or at the beginning of fermentation to maintain the pH between 5.5 and 6.0. The active fermentation is completed after 2–6 days, depending on the used-up carbon source concentration. The reaction involved in the production of calcium lactate during fermentation is in Equation (2). The produced calcium lactate determines the upper limit of sugar concentration. The calcium lactate produced is passed through the primary filter; the sludge is separated from calcium lactate, and the filtered calcium lactate goes into a decomposer tank.Lactic acid + Calcium carbonate → Calcium lactate + Water + Carbon dioxide
Calcium lactate + Sulphuric acid → Lactic acid + Gypsum
Lactic acid + Methanol → Methyl lactate + Water
Methyl lactate + Water → Lactic acid + Methanol
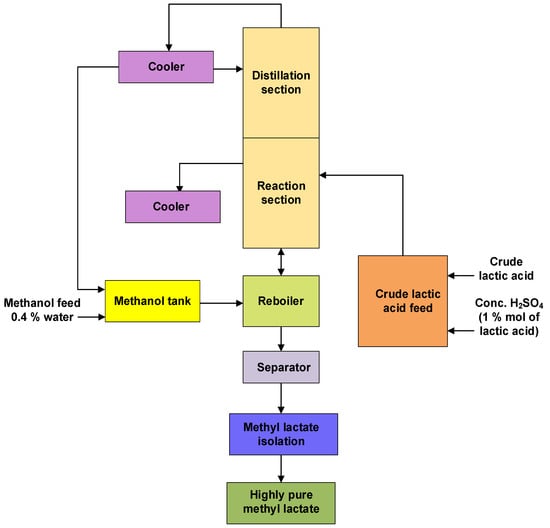
Figure 2. Block diagram for the second step of lactic acid purification that involves esterification of lactic acid with methanol in the presence of concentrated sulphuric acid forming methyl lactate (Adapted from Bapat et al. (2014) [21]).
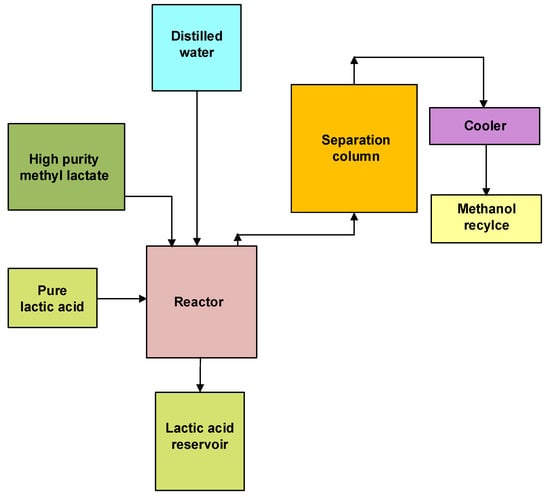
Figure 3. Block diagram for the third step of lactic acid purification involves hydrolysis of methyl lactate and activated carbon treatment (Adapted from Bapat et al. (2014) [21]).
3.2. Ammonium Lactate Process in Lactic Acid Production
The process steps in the ammonium lactate process are like that of the calcium lactate process, except that industrial fermentation is conducted using ammonia liquor. Ammonia used as a neutralizing agent in fermentative lactic acid production reduces the acidity of the fermentation broth and reacts with lactate to form ammonium lactate (Equation (6)) [1]. Lactic acid is recovered from ammonium lactate by acidulation with sulphuric acid, followed by ammonium sulfate salt crystallization (Equation (7)). Lactic acid can also be recovered from ammonium lactate by lactic acid esterification using alcohol and back recovery using pure distilled water or de-ionized water. Sun et al. (2006) [23] reported the extraction of lactic acid from a fermentation broth by esterification and hydrolysis; in the study, butanol reacts with ammonium lactate obtained directly from fermentation for 6 h to produce butyl lactate. Sun et al. (2006) [23] further stated that the cation exchange resin used in hydrolysis was modified by replacing sulphuric acid with stannous chloride as the catalyst; neutral ammonium lactate replaced lactic acid as the starting material, and the ammonium lactate was purified. The sequential hydrolysis of purified ammonium lactate in the presence of cation exchange resin in the H+ form for 4 h produced 89.7% lactic acid at a purity level of 90% [23].Lactic acid + Ammonia → Ammonium lactate
Ammonium lactate + Sulphuric acid → Lactic acid + Ammonia
References
- Vaidya, A.N.; Pandey, R.A.; Mudliar, S.; Kumar, M.S.; Chakrabarti, T.; Devotta, S. Production and Recovery of Lactic Acid for Polylactide—An Overview. Crit. Rev. Environ. Sci. Technol. 2005, 35, 429–467.
- Krishna, B.S.; Nikhilesh, G.S.S.; Tarun, B.; Saibaba, N.; Gopinadh, R. Industrial Production of Lactic Acid and Its Applications. Int. J. Biotechnol. Res. 2018, 1, 42–54.
- Yankov, D. Fermentative Lactic Acid Production from Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 1–34.
- Rodrigues, A.K.O.; Maia, D.L.H.; Fernandes, F.A.N. Production of Lactic Acid from Glycerol by Applying an Alkaline Hydrothermal Process Using Homogeneous Catalysts and High Glycerol Concentration. Braz. J. Chem. Eng. 2015, 32, 749–755.
- Ramírez-López, C.A.; Ochoa-Gómez, J.R.; Fernández-Santos, M.; Gómez-Jiménez-Aberasturi, O.; Alonso-Vicario, A.; Torrecilla-Soria, J. Synthesis of Lactic Acid by Alkaline Hydrothermal Conversion of Glycerol at High Glycerol Concentration. Ind. Eng. Chem. Res. 2010, 49, 6270–6278.
- Zhou, H.; Jin, F.; Wu, B.; Cao, J.; Duan, X.; Kishita, A. Production of Lactic Acid from C6-Polyols by Alkaline Hydrothermal Reactions. J. Phys. Conf. Ser. 2010, 215, 1–4.
- Buitelaar, M.M.; Van Daatselaar, E.; Van Teijlingen, D.G.; Stokvis, H.I.; Wendt, J.D.; De Sousa Ribeiro, R.J.; Brooks, A.M.M.; Kamphuis, E.C.; Lopez-Montoya, S.; Van Putten, J.C.; et al. Process Designs for Converting Propylene Glycol to Acrylic Acid via Lactic Acid and Allyl Alcohol. Ind. Eng. Chem. Res. 2020, 59, 1183–1192.
- Castillo-Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez, J.M.; Converti, G.A.; de Oliveira, R.P.S. Lactic Acid Properties, Applications and Production: A Review. Trends Food Sci. Technol. 2013, 30, 70–83.
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.-V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of Optically Pure Lactic Acid by Microbial Fermentation: A Review. Environ. Chem. Lett. 2020, 19, 539–556.
- Hofvendahl, K.; Hahn-Hägerdal, B. Factors Affecting the Fermentative Lactic Acid Production from Renewable Resources. Enzym. Microb. Technol. 2000, 26, 87–107.
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive Fermentation of Lactic Acid in Lactic Acid Bacteria Cultivation: A Review. Front. Microbiol. 2017, 8, 1–7.
- Martin, A.M. Fermentation Processes for the Production of Lactic Acid. In Lactic Acid Bacteria; Bozoglu, T.F., Ray, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 269–301.
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to Overcome the Current Limitations and Challenges for Efficient Microbial Production of Optically Pure Lactic Acid. J. Biotechnol. 2016, 236, 176–192.
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic Acid Fermentation from Food Waste with Indigenous Microbiota: Effects of PH, Temperature and High OLR. Waste Manag. 2016, 52, 278–285.
- Agblevor, F.A.; Evans, T.G. Method for Production of Lactic Acid. U.S. WO Patent US20040229327A1, 18 November 2004. Available online: https://patentimages.storage.googleapis.com/29/7b/58/d0fad015c210b8/US20040229327A1.pdf (accessed on 22 September 2022).
- Choudhary, J.; Singh, S.; Nain, L. Thermotolerant Fermenting Yeasts for Simultaneous Saccharification Fermentation of Lignocellulosic Biomass. Electron. J. Biotechnol. 2016, 21, 82–92.
- Wee, Y.J.; Yun, J.S.; Kim, D.; Ryu, H.W. Batch and Repeated Batch Production of L(+)-Lactic Acid by Enterococcus faecalis RKY1 Using Wood Hydrolyzate and Corn Steep Liquor. J. Ind. Microbiol. Biotechnol. 2006, 33, 431–435.
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic Acid Production from Lignocellulose-Derived Sugars Using Lactic Acid Bacteria: Overview and Limits. J. Biotechnol. 2011, 156, 286–301.
- Abedi, E.; Hashemi, S.M.B. Lactic Acid Production—Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6, 1–32.
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent Advances in Lactic Acid Production by Microbial Fermentation Processes. Biotechnol. Adv. 2013, 31, 877–902.
- Bapat, S.S.; Aichele, C.P.; High, K.A. Development of a Sustainable Process for the Production of Polymer Grade Lactic Acid. Sustain. Chem. Process. 2014, 2, 1–8.
- Barve, P.P.; Kulkarni, B.D.; Nene, S.N.; Shinde, R.W.; Gupte, M.Y.; Thite, G.A.; Chavan, V.B.; Deshpande, T.R. Process for Preparing L-(+)-Lactic Acid. U.S. WO Patent US2007010548A1, 25 January 2007. Available online: https://patentimages.storage.googleapis.com/bf/e3/2e/c864ae2338fb9c/WO2007010548A1.pdf (accessed on 2 October 2022).
- Sun, X.; Wang, Q.; Zhao, W.; Ma, H.; Sakata, K. Extraction and Purification of Lactic Acid from Fermentation Broth by Esterification and Hydrolysis Method. Sep. Purif. Technol. 2006, 49, 43–48.
- Idler, C.; Venus, J.; Kamm, B. Microbiology Monographs: Microorganisms in Biorefineries: Microorganisms for the Production of Lactic Acid and Organic Lactates Christine. In Microorganisms in Biorefineries; Kamm, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 26, pp. 225–273.
- Pleissner, D.; Neu, A.K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative Lactic Acid Production from Coffee Pulp Hydrolysate Using Bacillus Coagulans at Laboratory and Pilot Scales. Bioresour. Technol. 2016, 218, 167–173.
- Alexandri, M.; Schneider, R.; Venus, J. Membrane Technologies for Lactic Acid Separation from Fermentation Broths Derived from Renewable Resources. Membranes 2018, 8, 94.
- Kulprathipanja, S.; Oroskar, A.R. Separation of Lactic Acid from Fermentation Broth with an Anionic Polymeric Absorbent. U.S. WO Patent US5068418A, 26 November 1991. Available online: https://patentsgoogle.com/patent/US5068418A/en (accessed on 25 September 2022).
- Matsumoto, M.; Takahashi, T.; Fukushima, K. Synergistic Extraction of Lactic Acid with Alkylamine and Tri-n-Butylphosphate: Effects of Amines, Diluents and Temperature. Sep. Purif. Technol. 2003, 33, 89–93.
- Lee, H.D.; Lee, M.Y.; Hwang, Y.S.; Cho, Y.H.; Kim, H.W.; Park, H.B. Separation and Purification of Lactic Acid from Fermentation Broth Using Membrane-Integrated Separation Processes. Ind. Eng. Chem. Res. 2017, 56, 8301–8310.
- Din, N.A.S.; Lim, S.J.; Maskat, M.Y.; Mutalib, S.A.; Zaini, N.A.M. Lactic Acid Separation and Recovery from Fermentation Broth by Ion-Exchange Resin: A Review. Bioresour. Bioprocess. 2021, 8, 1–23.
- Pal, P.; Sikder, J.; Roy, S.; Giorno, L. Process Intensification in Lactic Acid Production: A Review of Membrane Based Processes. Chem. Eng. Process. 2009, 48, 1549–1559.
- Youcai, Z. Physical and Chemical Treatment Processes for Leachate. In Pollution Control ad Resource Recovery; Butterworth Heinemann; Elsevier: Oxford, UK, 2018; pp. 147–226.
More
