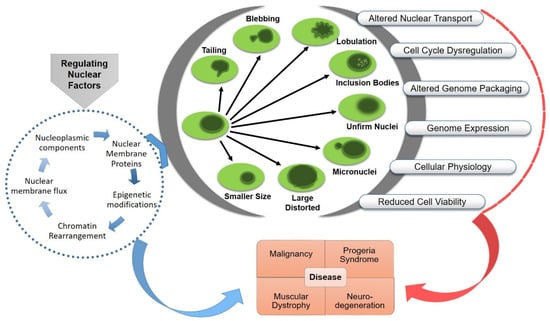
The organization of eukaryotic genome in the nucleus, a double-membraned organelle separated from the cytoplasm, is highly complex and dynamic. The functional architecture of the nucleus is confined by the layers of internal and cytoplasmic elements, including chromatin organization, nuclear envelope associated proteome and transport, nuclear–cytoskeletal contacts, and the mechano-regulatory signaling cascades. The size and morphology of the nucleus could impose a significant impact on nuclear mechanics, chromatin organization, gene expression, cell functionality and disease development. The maintenance of nuclear organization during genetic or physical perturbation is crucial for the viability and lifespan of the cell. Abnormal nuclear envelope morphologies, such as invagination and blebbing, have functional implications in several human disorders, including cancer, accelerated aging, thyroid disorders, and different types of neuro-muscular diseases.
- nuclear shape regulation
- nuclear size regulation
- nuclear envelope proteins
- nucleophagy
1. Introduction
2. Contribution of Nuclear Constituents in Regulation of Nuclear Morphology
The structural components of a nucleus, such as chromosomes, nucleoplasmic compartments, nuclear envelope proteins and lipid bilayer, are the core elements involved in the regulation of nuclear morphology. Each component has a distinct functionality and approach by which they help to maintain the characteristic shape and size of the nucleus.2.1. Nuclear Envelope Proteins
Nuclear envelopes are the structural and physiological interface between the central genomic material and cytoplasm of the cell. The double lipid membrane of the nuclear envelope originates from ER and remains in continuous contact with its network afterward. In contrast to the origin, both the outer nuclear membrane (ONM) and inner nuclear membrane (INM) of the nuclear envelope are enriched with a very distinguished set of proteomes (Figure 1) [14][15]. These subsets of proteins play key roles in bidirectional nucleoplasmic transportation, maintenance of nuclear architecture, cell cycle control, chromatin organization, gene regulation and DNA repair. The most complex macromolecular assemblies of the nuclear envelope are nuclear pore complexes (NPCs) [15][16][17][18][16,17,18,19]. NPCs encompass multiple subsets of more than 30 types of nuclear pore proteins called nucleoporins (Nups) [19][20][20,21]. The de novo assembly of Nups during interphase starts with the accumulation of Nups in both the outer and inner nuclear membranes, and the subsequent fusion these proteins forms the doughnut-shaped core (consisting of eight spokes arranged around a central channel) of NPC [21][22][23][22,23,24]. The fusion creates an energetically unfavorable and highly curved membrane that surrounds the NPC [24][25].

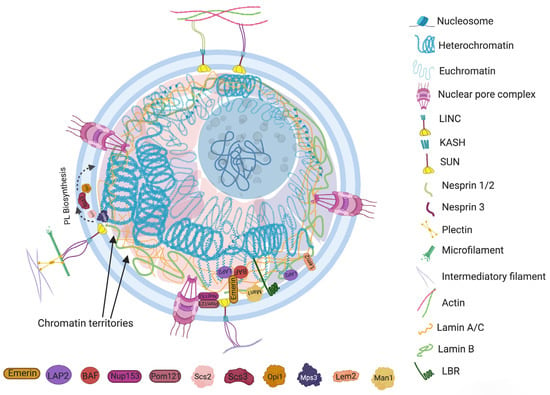
2.2. Nuclear Membrane Composition
2.3. Genome Organization
The organization of the genome within the nucleus is a nonrandom process. The second level arrangement of the genome contains euchromatin and constitutive or facultative heterochromatin that gives rise to some advanced assemblies, such as chromosome loops, topological associated domains (TADs, fundamental units of three-dimensional (3D) nuclear organization), lamin associated domains (LADs, heterochromatin located adjacent to lamina), nucleolar associated domains (NADs, heterochromatin located adjacent to the nucleolus) and chromosome territories. It is also known that the nuclear arrangement of chromatin is somehow related to the morphology of the nucleus [33][34][58,74]. The role of chromatin in sizing and shaping the nucleus is very intricate and diverse. However, it is widely understood that chromatin contributes to nuclear morphological regulation by (i) interacting with nuclear envelope via the LAD/NAD binding domains of INM integrated proteins and (ii) altering the biophysical properties of heterochromatin. In addition to nuclear envelope assembly, the biophysical state of constitutive and facultative heterochromatin largely influences the rigidity, shape and size of the nucleus [2][35][2,75]. Numerous studies have explored the role of ‘chromatin packing’ in nuclear morphology. A direct investigation was completed by Stephens et al. on chromatin decompaction of mammalian cells using histone deacetylase and histone methyltransferase inhibitors [2]. The study showed that an increase in the ratio of euchromatin caused softer nuclei and nuclear blebbing, which was independent of the involvement of lamins. The deformation was reversed after treating the cells with histone demethylase inhibitors. It was suggested that decompacted euchromatin might be mechanically weaker than heterochromatin, or that the altered chromatin state could cause a loss of chromatin lamina connection and nuclear rigidity [2].2.4. Nuclear Subcompartments and Nucleolus
Nucleoplasms are among the very eventful and crowded niche of the cell that provides a common working platform to several types of heterogeneous components. It includes chromatin attached proteins and other nuclear bodies, such as nucleoli, Cajal bodies, promyelocytic leukemia (PML) bodies, speckles, paraspeckles, polycomb bodies and histone locus bodies. The consortia of nuclear bodies combine to make the nuclear matrix, which is responsible for organizing different domains of chromatin fiber into the nuclear volume [36][78]. The microenvironment created by the concentration of specific proteins is referred to as membrane-less nuclear subcompartments (Figure 1). Some components of nuclear subcompartments could also contribute to the structural organization of the nucleus. For example, Morelli and coworkers have observed that the aberrant expression of heat shock protein B2 (HSPB2), which is a nuclear subcompartment protein, in myoblast cells could cause impaired LMNA-SUN2 anchoring at the nuclear envelope, thereby disrupting NE integrity [37][79]. The findings also stimulated reasonable thoughts about the impact of the nucleolus on nuclear morphology. The nucleolus is the most prominent nuclear subcompartment and covers almost one third of the nucleoplasm’s peripheral space. Its size varies during growth, and both normal and cancer cells proliferate due to the increased demand for ribosome biogenesis [38][39][40][81,82,83]. Almost any type of cancer exhibits abnormalities in the number and shape of nucleoli due to overactivated ribosome biosynthetic core machinery. However, it would be interesting to know whether nucleolus could have any influence on nuclear morphology in any of these conditions. The little research conducted on this topic have shown that a nucleolus has the ability to sequester the nuclear envelope to avoid nuclear morphological disruption [41][84]. The direct interaction between the nuclear envelope and nucleolus was explored by some researchers. A study on breast cancer cells revealed that depletion of the nuclear envelope protein SUN1 induced nucleolus enlargement [42][85]. It is already known that INM anchored and associated proteins contribute to maintaining nuclear envelope integrity and morphology. Sharing nuclear envelope proteins to maintain nucleolar and nuclear morphology was also observed by Sen Gupta and Sengupta [43][55].2.5. Nucleus and Cytoplasmic Components
The nucleus is a largest organelle and the center of essential genetic and regulatory activities of the eukaryotic cell. Constant physiological communication among the nucleus and other cellular components, such as mitochondria, ER, vacuoles, peroxisomes, plasma membrane, lipid droplets and cytosol, maintains the cellular homeostasis [44][45][86,87]. Strikingly, the direct physical interconnection involving specific tethering contacts has also been recognized among the membrane-bound organelles [46][88]. Since the nucleus is the largest and most vigorous organelle of the cell, the organization or reorganization of the cytoskeleton quickly transmits the cellular stress to the nucleus. For example, findings of Monroy-Ramírez and coworkers established that aberrant binding of tau protein and tubulin alters the radial organization of cytoskeleton to the thick ring type arrangement at peripheral and perinuclear sites [47][92]. The rehabilitated nuclear–cytoskeleton assembly causes enlargement and lobulation of the nucleus followed by functional abnormalities into the cell. The externally applied tension transfers to the nucleus via the actin filament anchoring LINC complex. The direct connection between actin cytoskeleton and nuclear morphology was observed in human melanoma cells by Colón-Bolea et al. [48][93]. The nuclear shape alteration in invasive melanoma cells was orchestrated by alteration in the connection between the tubulin cytoskeleton and LINC complex using a RHO GTPase (RAC1)-mediated mechanism [48][93]. The concept is further corroborated by Lu et al., who demonstrated the consequence of disruption in connection between a KASH motif containing proteins and an actin network [49][94]. A multivariate KASH motif containing protein, Nesprin, interacts with the actin cytoskeleton covering the outer nuclear membrane. The study revealed that Nesprin 1/ Nesprin 2 consists of a specific N-terminal actin binding domain (ABD) which is involved in actin mediated nuclear shape regulation. The overexpression of Nesprin 2 ABD leads to increase in nuclear area, but replacing it with a mini-isoform of Nesprin 2 that lacks the long rod segment produces smaller nuclei [49][94]. The scholars proposed that an interchain association of Nesprin produces a basket-like protein network which has a key role in effective transduction of nuclear and cytoplasmic forces. The nuclear shape is the net outcome of external (cytoskeleton) and internal (microfilaments, lamina, genome) generated forces from opposite sides of the nuclear envelope (Figure 2).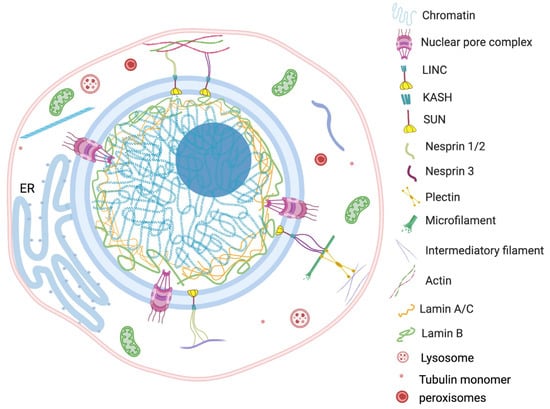
3. Functional Consequences of Nuclear Aberrations