Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yukihito Higashi and Version 2 by Rita Xu.
Oxidative stress and chronic inflammation play an important role in the pathogenesis of atherosclerosis. Atherosclerosis develops as the first step of vascular endothelial dysfunction induced by complex molecular mechanisms. Vascular endothelial dysfunction leads to oxidative stress and inflammation of vessel walls, which in turn enhances vascular endothelial dysfunction. Vascular endothelial dysfunction and vascular wall oxidative stress and chronic inflammation make a vicious cycle that leads to the development of atherosclerosis.
- oxidative stress
- inflammation
- endothelial function
1. Introduction
Oxidative stress and inflammation play an important role in the development of cardiovascular diseases (CVD) [1][2][3][4][5][1,2,3,4,5]. The sources of production of reactive oxygen species (ROS) in blood vessels include nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, uncoupled endothelial nitric oxide synthase (eNOS), arachidonic acid metabolic pathway, mitochondrial electron transfer system dysfunction, and catecholamine autoxidation [6][7][6,7]. Among these, NADPH oxidase is the most important and is involved in the production of ROS in vivo, and it is activated by various cytokines, vasoactive substances, and shear stress [8][9][8,9]. It is well known that ROS have opposing effects on cell viability. ROS induce cell proliferation, hypertrophy, migration, and apoptosis [10][11][10,11]. Chronic inflammation, which begins as a biological response to vascular endothelial dysfunction, is also thought to be the primary cause of atherosclerosis [3][4][5][3,4,5]. Various factors including oxidative stress, oxidized low-density lipoprotein (LDL), thrombi, and viral and bacterial infections induce acute and chronic inflammatory cell infiltrates, including neutrophils, lymphocytes, and macrophages, which in turn enhance local vascular inflammation by enhancing the production of inflammatory cytokines by the infiltrating inflammatory cells [12][13][12,13]. It is thought that in the presence of CVD, chronic inflammation makes a vicious cycle, leading to the maintenance and progression of atherosclerosis. Therefore, evaluation of vascular function, which is the primary point of action of chronic inflammation, is extremely important for elucidating the pathogenesis of CVD, determining therapeutic efficacy, and predicting prognosis.
2. Vascular Endothelial Function
The vascular endothelium is anatomically located in the innermost layer of blood vessels, and the vascular system, a closed system consisting of the heart, arteries, and veins, is composed entirely of one cell layer. Endothelium cells secrete vasodilators including nitric oxide (NO), prostaglandin I2, C-type natriuretic peptide, and endothelium-derived vascular hyperpolarizing factor as well as vasoconstrictors including endothelin, angiotensin II, prostaglandin H2, and thromboxane A2 [14][15][14,15]. Among these bioactive substances, NO plays a very important role in atherosclerosis. NO is produced and secreted from L-arginine, an essential amino acid, by activation of endothelial NO synthase (eNOS) through mechanical stimulation of vascular endothelial cells or binding to receptors by agonists such as acetylcholine, histamine, and bradykinin or by shear stress from blood flow in vascular endothelial cells [16][17][16,17]. The secreted gaseous NO is transmitted by diffusion to nearby VSMCs and activates intracellular soluble guanylate cyclase, which increases the amount of cyclic guanosine monophosphate and relaxes vascular smooth muscle [18]. The normal vascular endothelium maintains vascular function and structure by adjusting the balance between vasodilation and vasoconstriction, proliferation and antiproliferation of VSMCs, coagulation and anticoagulation, inflammation and anti-inflammation, and oxidation and antioxidation [19]. If all of the vascular endothelium in the body could be collected, the total weight would be equivalent to that of the liver. If it could be spread over an entire surface, the total area would be equivalent to six tennis courts and if it could be connected in a row, the length would be 100,000 km or two and a half times around the earth [20]. Atherosclerosis develops and progresses the first stage of vascular endothelial dysfunction [20]. Hypertension, diabetes, dyslipidemia, aging, obesity, smoking, lack of exercise, excessive salt intake, and menopause induce vascular endothelial dysfunction [21]. Aging is the largest factor that determines vascular endothelial function [22]. Vascular endothelial function is now recognized as a predictive factor in the development of CVD. A meta-analysis by Lerman et al. [23] confirmed that endothelial function is an independent predictor of cardiovascular complications. Vascular endothelial function can also be viewed as a target for treatment of atherosclerosis. Endothelial dysfunction is not irreversible and can be corrected with appropriate interventions such as antihypertensive medications, antidiabetic agents, lipid-lowering therapy, and lifestyle modification [24][25][26][27][28][29][30][24,25,26,27,28,29,30]. Improvement in endothelial dysfunction is expected to reduce the incidence of coronary and cerebrovascular events and improve life expectancy in the future. Figure 1 shows the roles of oxidative stress and inflammation in the process of endothelial dysfunction-related disease.
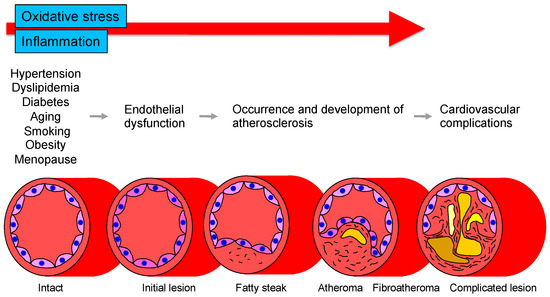
Figure 1. Roles of oxidative stress and inflammation in the process of endothelial dysfunction-related disease.
Several methods for measuring vascular endothelial function are currently used [31][32][31,32]. These can be broadly classified into methods that evaluate blood flow and vessel diameter by reactive hyperemia after ischemia and methods that evaluate reactivity by administering a bioactive substance. Unfortunately, there is no gold standard method, but at this stage, plethysmography is considered to best reflect vascular endothelial function. In general, this method evaluates vascular endothelial function by measuring changes in blood flow through selective administration of NO-producing stimulants such as acetylcholine, methacholine, bradykinin, histamine, or NO inhibitors to arteries in the limbs. Various vasoactive substances can be used in this method in addition to NO agonists and antagonists, allowing multifaceted evaluation of the dynamics of various bioactive substances produced and secreted by vascular endothelial cells, rather than examining only NO from a single perspective. The ultrasound-based method, flow-mediated vasodilation (FMD), is evaluated by the change in vessel diameter after reactive hyperemia in an extremity. FMD (% change) is calculated by [(maximum vessel diameter after lifting of the frame − baseline vessel diameter)/baseline vessel diameter] × 100. Measurement of peripheral arterial pulse amplitude in fingers is also used to assess endothelial function. The ratio of peripheral arterial pulse amplitude before and after reactive hyperemia (reactive hyperemia index) is calculated. Measurement of biomarkers in blood or urine is the most convenient and non-invasive method, but, unfortunately, there are currently no biomarkers that can be evaluated. However, there are various problems such as the possibility that they do not directly reflect NO production and the accuracy of measurement. These measurements should be considered as adjuncts to endothelial function assessment using FMD or plethysmography. FMD measurement is currently the most widely used method for evaluating vascular endothelial function and is likely to become more widely used in the future. However, it is also true that the method still has many issues to be resolved and many problems to be solved in the future, including the issue of reproducibility. Each method has its own advantages and disadvantages, and it is desirable to improve, refine, and standardize the method or to develop a new concept of a measurement device [32].
3. Oxidative Stress
3.1. Oxidative Stress and Vascular Injury
Oxidative stress plays an important role in CVD and CV events through various mechanisms including activation of NADPH oxidase, xanthine oxidase and cyclooxygenase, dysfunction of the mitochondrial electron transfer system, uncoupled eNOS, catecholamine autoxidation and failure of the antioxidative system (Figure 2). Circulating LDL enters the subendothelium and undergoes denaturation by oxidative stress to become oxidized LDL [12][13][12,13]. Oxidized LDL induces the expression of monocyte migration factor macrophage chemotactic protein 1 (MCP-1) in vascular endothelial cells and also induces the expression of adhesion factors such as intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), causing peripheral monocytes to adhere to the endothelium and invade the subendothelium [33]. Oxidized LDL also induces the secretion and production of macrophage colony-stimulating factor by vascular endothelial cells, leading to the maturation and differentiation of monocytes into macrophages. Oxidized LDL induces apoptosis in many cells, but it has been observed that a large amount of oxidized LDL accumulates in macrophages, leading to foam cell formation without inducing apoptosis [34]. It is thought that these foam cells release various cytokines, inducing inflammation and oxidative stress in the vascular endothelium. In atherosclerotic lesions, these systems make a vicious cycle that leads to the formation of atheroma and, in the final stage, to CV events. Oxidized LDL has also been reported to promote apoptosis of VSMCs lining the luminal side of the atheroma (thinning of the fibrous membrane), which may contribute to atheroma fragility and instability [35]. Lysophosphatidylcholine produced during oxidative degeneration of LDL impairs the NO synthesis system and contributes to the decrease in NO production [36]. Oxidized LDL is also known to act in thrombus formation by increasing plasminogen activator inhibitor-1 (PAI-1) production and decreasing tissue plasminogen activator (t-PA) production from vascular endothelial cells [37]. Thus, oxidized LDL is widely involved in the initiation, maintenance, and progression of atherosclerosis and atherogenesis. Lectin-like oxidized LDL receptor-1 (LOX-1), a novel scavenger receptor for oxidized LDL, was cloned in vascular endothelial cells [38]. In vitro, LOX-1 expression is known to be induced by various cytokines and shear stress [39]. Interestingly, LOX-1 is rarely expressed in normal vessels but is strongly expressed in endothelial cells, VSMCs and macrophages in atherosclerotic lesions [39][40][39,40].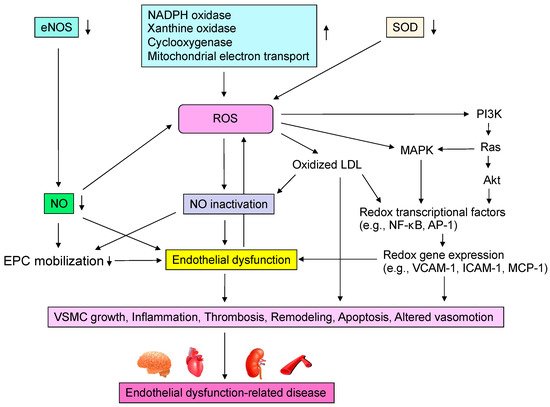
Figure 2. Mechanisms by which reactive oxygen species (ROS) induce endothelial dysfunction-related disease. eNOS, endothelial nitric oxide synthase; NO, nitric oxide; NADPH, nicotinamide adenine dinucleotide phosphate; SOD, superoxide dismutase; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase kinases; NF-κB, nuclear factor-kappa B; AP-1, activator protein-1; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1; MCP-1, macrophage chemotactic protein 1; EPC, endothelial progenitor cell; VSMC, vascular smooth muscle cell.
