Selenium is one of the eight necessary trace elements humans require for active health balance. It contributes in several ways to the proper functioning of selenoprotein. Selenium has received enormous interest recently due to its therapeutic potential against a number of ailments. NTo date, numerous chemical compounds containing selenium have been investigated for the therapy of cancer and other disorders. Unifying the selenium atom into chemical components (typically organic) greatly increased their bioactivities. Selenocysteine can substitute the effect of cysteine and shield healthy cells from the adverse effects of reactive oxygen species (ROS); in other ways, specificWe foresee that the structure–property relationship of recently developed materials could significantly decrease the laborious work of background research to achieve target-oriented drug design in coming years. This review summarizes the research progress in the last 10 to 15 years and the application of selenium-containing compounds are classified as antioxidant agents that preserve the redox environment in healthy cellin the design and synthesis of those materials for potential antioxidant and anticancer agents.
1. Introduction
Selenium is a nonmetal/metalloid with characteristics halfway between those of sulfur and tellurium
[1][2][1,2]. In the Earth’s crust, it hardly ever exists in either its elemental state or as a pure ore complex. Jöns Jacob Berzelius made the discovery of selenium in 1817 and remarked that it resembled the already-known element tellurium
[1]. Although minute amounts of selenium are required for normal cellular function, elemental selenium and selenium salts are hazardous in even small levels and can lead to selenosis
[3]. The antioxidants glutathione peroxidase and thioredoxin reductase, as well as deiodinase enzymes, contain selenium, which is listed as an element in many multivitamins and other nutritional supplements
[4][5][4,5]. Thioredoxin reductase and glutathione peroxidase catalyze processes that are necessary for shielding biological components from oxidative and free radical damage. The amount of selenium needed by different plant species varies from high levels to zero level
[6]. The current recommended daily selenium allowance or recommended dietary allowances (RDA) for adult men and women is 55 micrograms
[7].
Selenium-based heterocycles have made a valuable contribution in material science due to their electron-donating and electron-accepting abilities and their ability to modify electronic, structural, and morphological properties
[8][9][10][11][8,9,10,11]. Selenium-based heterocycles have been utilized in small molecules, oligomers, and polymers to improve the electronic properties in an incremental direction
[12][13][14][15][12,13,14,15]. Multiple selenium-containing compounds
[12][16][12,16] have also been developed to improve the electronic behavior with higher mobility value in field-effect transistor (FET) devices
[17]. They have also been used as polymers in organic solar cells and organic electronic devices for better power conversion efficiency (PCE) and mobility value for implementation purposes
[18].
Selenium-containing compounds have been of great significance in the domain of medicinal chemistry. Selenium and selenium-containing compounds continue to be topics of substantial interest in the fields of biochemistry, epidemiology, and pharmacology. Numerous diseases that are now known to be linked to selenium deficiency have historically affected specific groups
[19][20][19,20]. When considering medicinal uses, it is vitally important to manage the right amount and the right molecular types of selenium-based pharmaceuticals. It is important to note that more than 40 years ago, the idea that selenium might have a preventive effect against human cancer was explored in mainstream scientific literature
[21][22][21,22]. Cancer death rates were found to be much lower in US counties with moderate or high selenium levels, as opposed to counties with low selenium levels, which revealed an inverse relationship between selenium levels and cancer incidence
[23]. Low selenium levels have been perceived in breast cancer patients and patients with pancreatic carcinomata. Selenium compounds have been shown to have antitumorigenic properties in several animal studies
[24].
As a crucial trace element, selenium also performs a number of exclusive activities and has significant metabolic impacts on human health
[25]. Numerous clinical studies have demonstrated that selenium deficit in the human body can lead to many significant life-threatening disorders, such as cancer, liver disease, organ failure, and cardiovascular disease
[26][27][26,27]. Several studies have demonstrated that individuals with low or deficient selenium levels require an appropriate selenium supplement, which helps in boosting antioxidant activity and augmenting cellular defense capability
[28]. As a result, the development of unique selenium compounds provides a desirable toolset for medical chemists in building effective pharmaceuticals. Selenium-containing compounds have been found to have antioxidants, anticancer agents, and antiparasitic, antibacterial, antiviral, antifungal, and neuroprotective properties
[24][29][30][24,29,30].
2. Antioxidant Activity
Selenocysteine can substitute the effect of cysteine and shield healthy cells from the adverse effects of reactive oxygen species (ROS); in other ways, specific selenium compounds (
Figure 1) are classified as antioxidant agents that preserve the redox environment in healthy cells
[31]. In contrast, oxidation occurs at advanced levels in cancer cells and affects various aspects of their activity with ineffective antioxidant mechanisms
[32]. Selenium can be administered in its elemental form or after combining it with other inorganic or organic compounds, where low doses promote cell development and high levels exhibit a cytotoxic impact
[32].
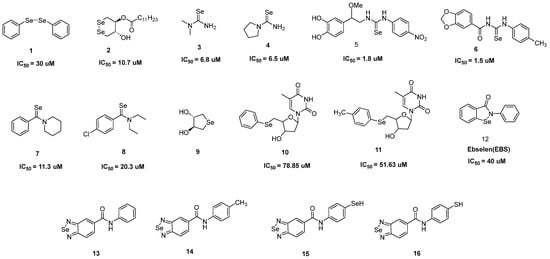
Selenium-containing compounds with antioxidant activity [31,32,33,34,35,36,37,38,39,40,41,42,43].
The excellent antioxidant and glutathione-peroxidase-like properties of diphenyl diselenide
1 help to defend macrophages from atherogenic signaling. Pretreating J774A.1 macrophages with
1 substantially reduced the formation of ROS driven by oxidized low-density lipoprotein (oxLDL)
[33]. Without affecting by inherent cytotoxicity,
1 was able to prevent breast cancer (MCF-7) cells from oxidative damage imposed by tamoxifen
[34]. Cyclic diselenide, compound
2, outperformed in terms of anti-lipid peroxidation activity (IC
50 = 10.7 μM)
[35]. In actuality, the coexistence of dithiothreitol [Se⁻ Se
−] caused by the diselenide (Se-Se) bond reduction resulted in highly active [Se
−, Se
−] that helped to convert H
2O
2 to H
2O and reintroduce the original Se-Se form
[36]. Moreover, Se-Se directly converted the lipid peroxide to the respective alcohol and inhibited the chain reaction of free radicals as an antioxidant in the process of lipid peroxidation of lecithin/cholesterol liposomes caused by 2,2′-azobis (2-aminopropane) dihydrochloride (AAPH).
Dimethylselenourea
3 and its pyrrolidine analog
4 with IC
50 values of 6.8 and 6.5 μM, respectively, showed antioxidant behavior and rapidly scavenged O
2− produced by polymorphonuclear leukocyte (PMNs). Selenoureas appeared to readily scavenge O
2− and generate O
2, with little to no NADPH oxidase activity inhibition. Selenourea analog
5 also showed excellent anti-free radical properties and acted as a biomimetic catalyst for glutathione peroxidase to scavenge H
2O
2 (IC
50 value of 3.76 μM on HeLa cells)
[37]. Similarly, selenourea-based compound
6 demonstrated strong radical scavenging activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylenebenzothiazoline)-6-sulfonic acid (ABTS) assays and had a preventive role on cells against oxidative stress
[38]. Phenyl-ring-containing selenamides
7 and
8, with IC
50 values of 11.3 and 20.3 μM, respectively, efficiently scavenged O
2⁻ from PMNs
[39].
With good water solubility, cycloselenide
9 catalyzed the dielectronic reduction of H
2O
2 to H
2O and the oxidation of mercaptan (RSH) to disulfide (RSSR) through a catalytic cycle akin to the GPX
[40]. The stimulation of the apoptotic signaling pathway in healthy cells was caused by a disparity in redox and immunological homeostasis initiated by the zidovudine derivatives
10 and
11 [41]. Ebselen (EBS) was demonstrated to be a glutathione peroxidase mimic and a peroxynitrite scavenger
[42]. EBS pretreatments significantly reduced the amount of thiobarbituric acid-induced substance production on mouse skin. Leukocyte infiltration and activation were specifically prevented by EBS, which reduced the H
2O
2 level. Compounds
13,
14, and
15 were capable of neutralizing the DPPH activity by 20% at 0.25 mg/mL, whereas compound
16 scavenged the DPPH activity by >40% at 0.25 mg/mL. The result indicated that the selenadiazoles ability to scavenge free radicals was concentration-dependent
[43].