Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Anastasia Bougea.
The relationship between migraine and tension-type headache (TTH) with Parkinson’s disease (PD) is controversial, while a common pathophysiological link remains obscure. The aim of thires systematic reviewearchers is to investigate the association between PD, migraine and TTH.
- Parkinson’s disease (PD)
- migraine
- tension-type headache (TTH)
- non-motor symptoms (NMS)
1. Introduction
Although Parkinson’s disease (PD) is classified as a motor neurodegenerative disorder, non-motor symptoms (NMSs)are common, such as sleep disorders, depression, cognitive deficits and pain [1]. Among PD patients, different pain phenotypes and/or classification shave been described: PD-related (including fluctuation-related, dyskinesia -related and central pain) vs. non-PD-related pain; primary vs. secondary (musculoskeletal system) pain; nociceptive vs. neuropathic; chronic vs. acute; and pain according to different body parts [2,3,4,5][2][3][4][5]. However, the exact pain etiology in PD remains unclear; as a consequence, therapeutic options are often ineffective [6].
Primary headaches (i.e., migraine and tension-type headache (TTH)) are commonpain disorders with several comorbidities, such as cardiovascular disease, stroke, depression and various dementias, and are accompanied by a substantial socioeconomic impact [7,8][7][8].Over the previous two decades, several studies have suggested a link between primary headaches and PD [9,10[9][10][11],11], while others have produced conflicting results [12,13][12][13]. Barbanti et al. identified a lifetime migraine prevalence of 27.8% and a current migraine prevalence of 13.1% in a population of PD patients [14]. Moreover, a cohort study showed that subjects with a midlife history of headache and, particularly, those with migraine with aura had an increased likelihood of PD [9].By contrast, Nunes et al. [15] demonstrated that PD patients had a lower lifetime prevalence of headache than controls. Previous studies have focused on the relationship between migraine and PD, and a prior validation study reported that 80% of individuals with non-migrainous headache might have TTH [16]. However, the supposed overlapping PD and headache pathophysiological mechanisms remain obscure [17]. Despite the high prevalence of migraine across various PD populations [14[14][15],15], headache remains an underestimated and undertreated NMS contributing to a poor quality of life among PD patients [14].
A clarification of the examined relationship will provide valuable knowledge to neurologists in determining the modifiable causes of poor outcomes.
2. Headache Prevalence in PD Patients
The overall pooled prevalence of any headache (including migraine and TTH) among PD patients was 49.1% (5 studies; 760 PD patients; 40.9%; 49.1% with 95% CI 24.8–73.6; and I2 = 97.3%, Figure 21). The 12-month prevalence of migraine among PD patients was calculated at 13.1% (3 studies; 771 PD patients; 11.2%; 13.1% with 95% CI 3.8–26.2; and I2 = 94.1%), while the lifetime prevalence was expectedly higher at 15.7% (5 studies; 1144 PD patients; 14.5%; 15.7% with 95% CI 7.9–25.2; and I2 = 92.3%, Figure 21). Only very few PD patients had received a diagnosis of migraine with aura (3 studies; 719 PD patients; 2.6%; 0.2% with 95% CI 0.0–8.6; and I2 = 93.5%).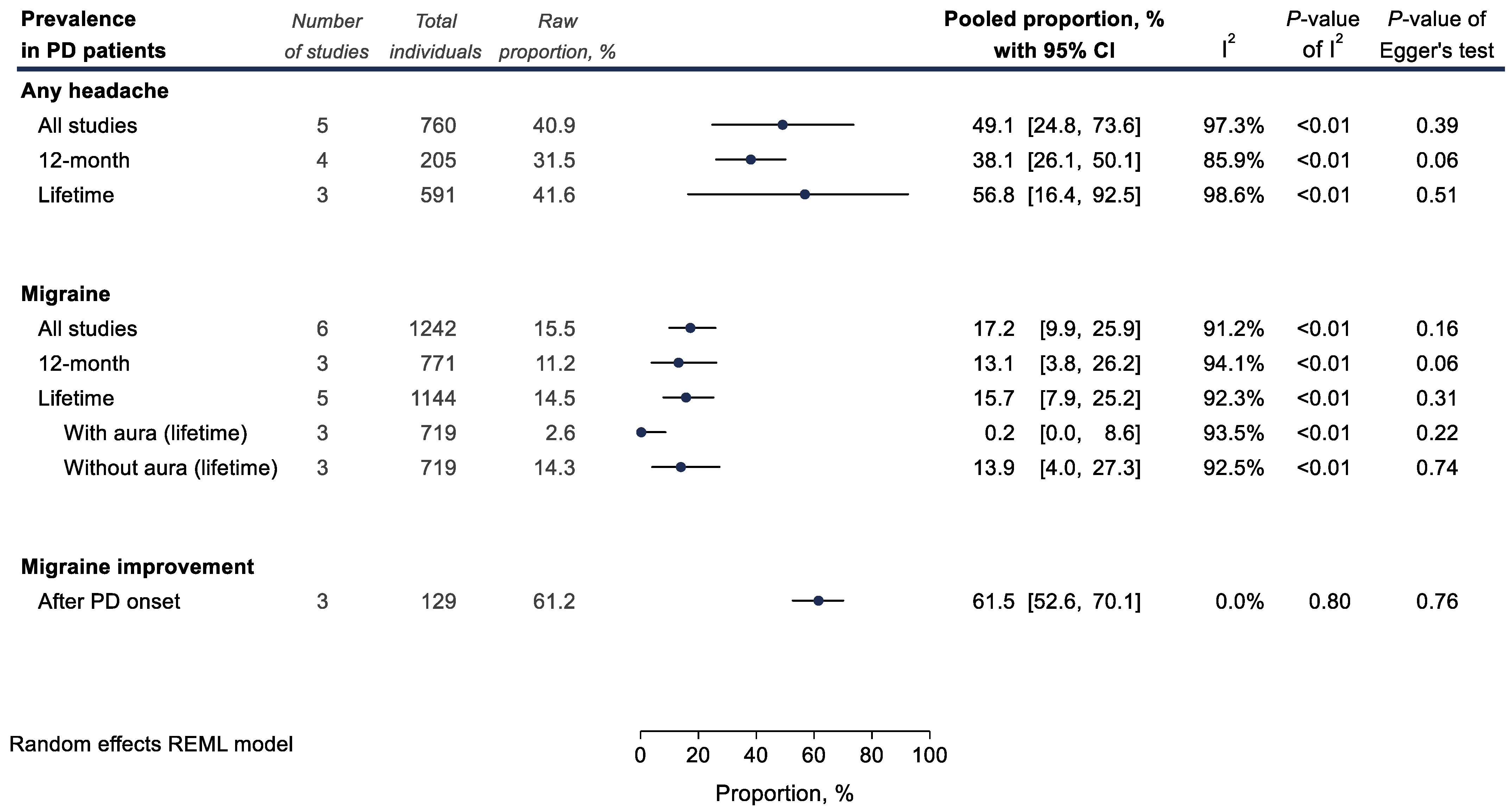
Figure 2. Meta-analyses of proportions examining headache prevalence (%) among Parkinson’s disease (PD) patients. Results of each individual random effects meta-analysis are depicted as black data markers; 95% confidence intervals (CI) are indicated by the black error bars. I2 reflects each meta-analysis heterogeneity.
3. Association between PD and Migraine
Overall, regarding the relationship between PD and migraine, no statistically significant association was found (7 studies; 302,165 individuals; ORpooled = 1.11, 95% CI 0.72–1.72; and I2 = 92.0%; Figure 32).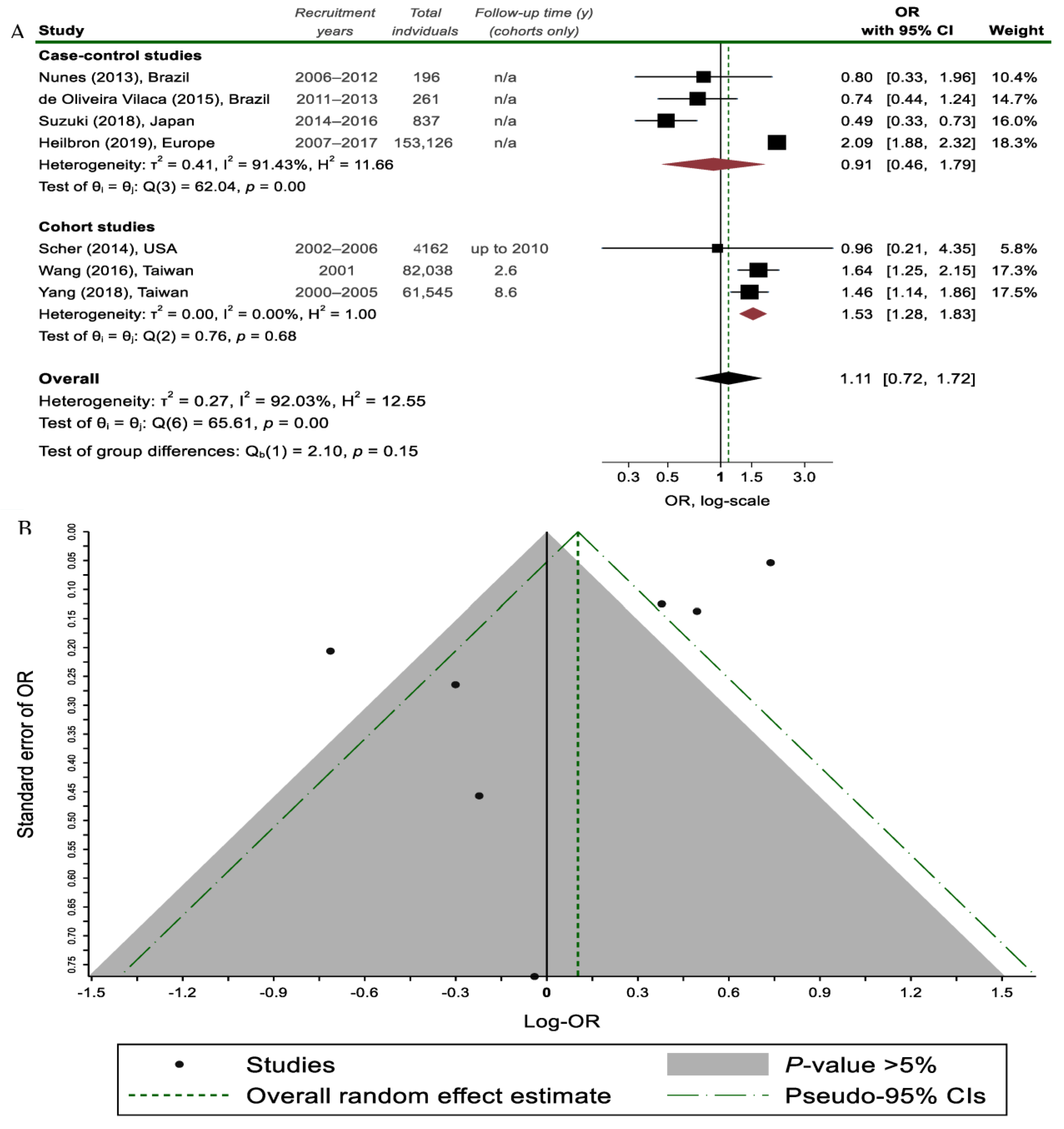
Figure 32. (A) Random effects meta-analysis on the association between migraine headaches and Parkinson’s disease (PD) status stratified by study design; (B) Funnel plot, where each study is depicted as a dot. The light grey shaded area contains studies with non-significant results (p > 0.05) [7,9,15,22,27,31,32][7][9][15][18][19][20][21].
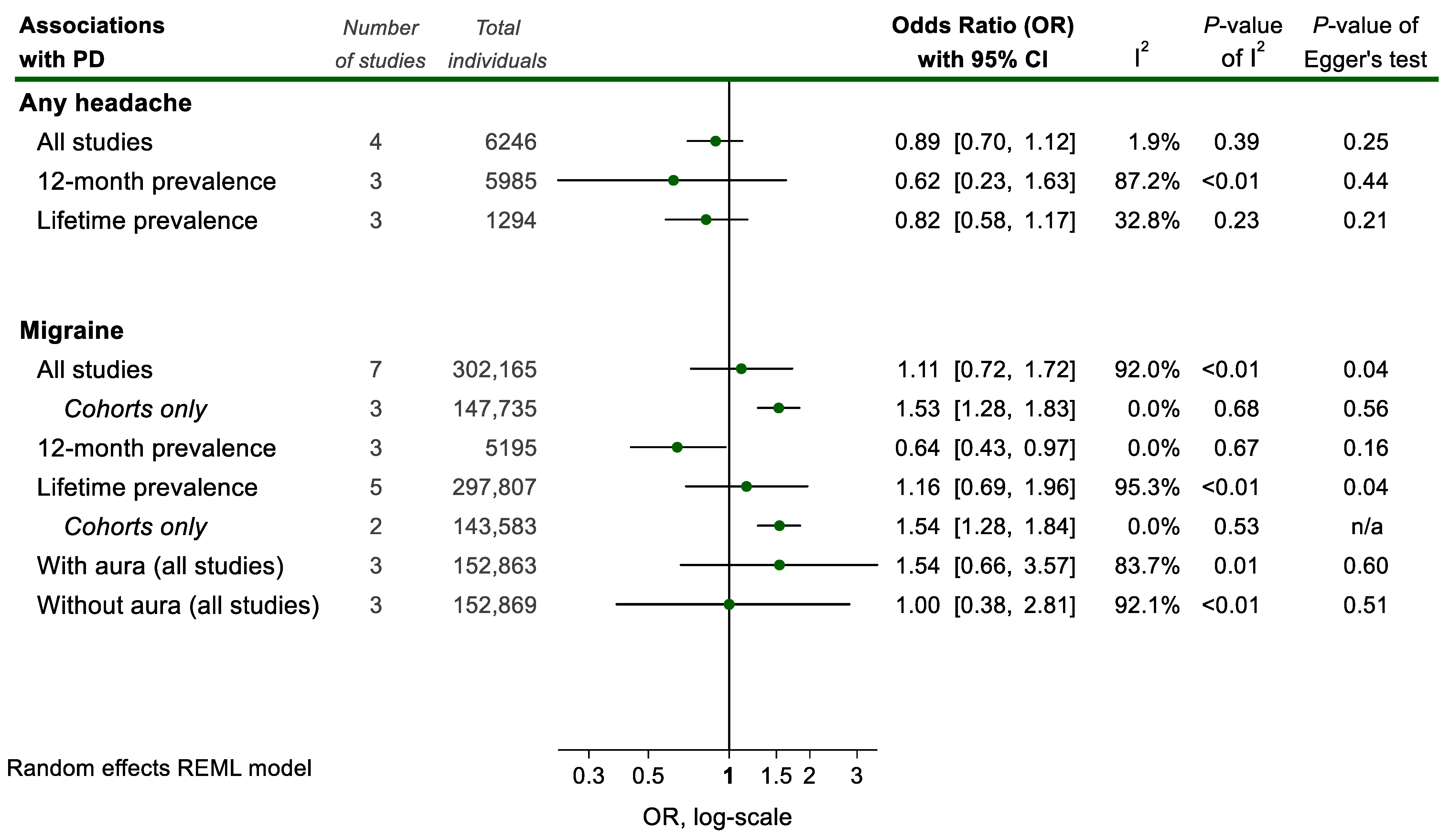
Figure 43. Meta-analyses of associations between headache and Parkinson Disease (PD) status. Results of each individual random effects meta-analysis (odds ratios (OR)) are depicted as green data markers; 95% confidence intervals (CI) are indicated by the black error bars. I2 reflects each meta-analysis heterogeneity.
4. Association between PD and TTH
Only 3 studies [15,22,30][15][18][22] examined the prevalence and/or associations between TTH and PD with conflicting results. WResearchers were unable to pool this data due to the vastly different methodological designs of the respective studies. Yang et al. suggest that TTH suffering individuals are at an increased risk of incident PD later in life. This is in line with the findings from a small PD patient sample by Sampaio Rocha-Filho et al. [30][22], where TTH prevalence among PD individuals was high (67.4%). On the contrary, Nunes et al. [15] suggest that PD is associated with a lower TTH 12-month prevalence. These results are directionally similar with the ones pertaining to the PD-migraine association.5. Genetics and Family History of Migraines in PD Patients
Whether genetics and a family history of migraines in PD patients influences the course of PD is not clear, as relevant data are reported in only 3 studies [13,14,22][13][14][18]. The course of migraine after the onset of PD in patients without a family history of migraine differed to that of those with a family history [22][18]. PD onset seemed to alter the course of migraine among migraineurs without a family history of migraine but not among those with a self-reported family history. [13]. However, Barbanti et al. [14] indirectly refute this finding, as almost half of PD patients without a family history of migraine had current migraine. Moreover, the lower prevalence of family history of migraine among PD migraineurs could not be explained.References
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22 (Suppl. S1), S119–S122.
- Antonini, A.; Tinazzi, M.; Abbruzzese, G.; Berardelli, A.; Chaudhuri, K.R.; Defazio, G.; Ferreira, J.; Martinez-Martin, P.; Trenkwalder, C.; Rascol, O. Pain in Parkinson’s disease: Facts and uncertainties. Eur. J. Neurol. 2018, 25, 917-e69.
- Young Blood, M.R.; Ferro, M.M.; Munhoz, R.P.; Teive, H.A.G.; Camargo, C.H.F. Classification and Characteristics of Pain Associated with Parkinson’s Disease. Park. Dis. 2016, 2016, 6067132.
- Wasner, G.; Deuschl, G. Pains in Parkinson disease--many syndromes under one umbrella. Nat. Reviews. Neurol. 2012, 8, 284–294.
- Ford, B. Pain in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25 (Suppl. S1), S98–S103.
- Buhmann, C.; Kassubek, J.; Jost, W.H. Management of Pain in Parkinson’s Disease. J. Park. Dis. 2020, 10, S37–S48.
- Wang, J.; Xu, W.; Sun, S.; Yu, S.; Fan, L. Headache disorder and the risk of dementia: A systematic review and meta-analysis of cohort studies. J. Headache Pain 2018, 19, 95.
- Caponnetto, V.; Deodato, M.; Robotti, M.; Koutsokera, M.; Pozzilli, V.; Galati, C.; Nocera, G.; De Matteis, E.; De Vanna, G.; Fellini, E.; et al. Comorbidities of primary headache disorders: A literature review with meta-analysis. J. Headache Pain 2021, 22, 71.
- Scher, A.I.; Ross, G.W.; Sigurdsson, S.; Garcia, M.; Gudmundsson, L.S.; Sveinbjörnsdóttir, S.; Wagner, A.K.; Gudnason, V.; Launer, L.J. Midlife migraine and late-life parkinsonism: AGES-Reykjavik study. Neurology 2014, 83, 1246–1252.
- Wang, H.I.; Ho, Y.C.; Huang, Y.P.; Pan, S.L. Migraine is related to an increased risk of Parkinson’s disease: A population-based, propensity score-matched, longitudinal follow-up study. Cephalalgia Int. J. Headache 2016, 36, 1316–1323.
- Meco, G.; Frascarelli, M.; Pratesi, L.; Linfante, I.; Rocchi, L.; Formisano, R. Headache in Parkinson’s disease. Headache 1988, 28, 26–29.
- Lorentz, I.T. A survey of headache in Parkinson’s disease. Cephalalgia Int. J. Headache 1989, 9, 83–86.
- van Hilten, J.J. The migraine-dopamine link: Do migraine and Parkinson’s disease coexist? Clin. Neurol. Neurosurg. 1992, 94, 168–170.
- Barbanti, P.; Fabbrini, G.; Vanacore, N.; Rum, A.; Lenzi, G.L.; Meco, G.; Cerbo, R. Dopamine and migraine: Does Parkinson’s disease modify migraine course? Cephalalgia Int. J. Headache 2000, 20, 720–723.
- Nunes, J.C.; Costa Bergamaschi, E.N.; Freitas, F.C.; Diaz, A.P.; Queiroz, L.P.; Debona, R.; Prediger, R.D.; Linhares, M.N.; Lin, K.; Walz, R. Prevalence of headache in patients with Parkinson’s disease and its association with the side of motor symptom onset. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014, 35, 595–600.
- Hagen, K.; Zwart, J.A.; Aamodt, A.H.; Nilsen, K.B.; Bråthen, G.; Helde, G.; Stjern, M.; Tronvik, E.A.; Stovner, L.J. The validity of questionnaire-based diagnoses: The third Nord-Trøndelag Health Study 2006–2008. J. Headache Pain 2010, 11, 67–73.
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G. Dopaminergic symptoms in migraine. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2013, 34 (Suppl. S1), S67–S70.
- Yang, F.C.; Chen, H.J.; Lee, J.T.; Chen, S.J.; Sung, Y.F.; Kao, C.H.; Yang, T.Y. Increased risk of Parkinson’s disease following tension-type headache: A nationwide population-based cohort study. Oncotarget 2018, 9, 2148–2157.
- Heilbron, K.; Noyce, A.J.; Fontanillas, P.; Alipanahi, B.; Nalls, M.A.; Cannon, P. The Parkinson’s phenome-traits associated with Parkinson’s disease in a broadly phenotyped cohort. NPJ Park. Dis. 2019, 5, 4.
- de Oliveira Vilaça, C.; Leite, M.A.; de Souza, J.A.; Orsini, M.; Pereira, J.S.; Amaral, C. The Behavior of Migraine in Patients with Parkinson’s Disease. Neurol. Int. 2015, 7, 6133.
- Suzuki, K.; Okuma, Y.; Uchiyama, T.; Miyamoto, M.; Sakakibara, R.; Shimo, Y.; Hattori, N.; Kuwabara, S.; Yamamoto, T.; Kaji, Y.; et al. The prevalence, course and clinical correlates of migraine in Parkinson’s disease: A multicentre case-controlled study. Cephalalgia Int. J. Headache 2018, 38, 1535–1544.
- Sampaio Rocha-Filho, P.A.; Leite Souza-Lima, C.F. Parkinson’s Disease and Headaches: A Cross-Sectional Study. Headache 2020, 60, 967–973.
More
