Binding of the immune checkpoint programmed cell death protein 1 (PD-1) to its ligand programmed death-ligand 1 (PD-L1) downregulates the adaptive immune response. PD-L1 is regularly expressed by antigen presenting cells. During an acute immune response, effector T cells transiently upregulate PD-1. In contrast, chronic immune stimulation leads to continuous expression of PD-1 on effector T cells. The latter also occurs in the tumor microenvironment, where PD-L1 can be expressed by tumor cells. The PD-1/PD-L1 pathway is an excellent example for the clinical application of biomarker research in the context of comparative immuno-oncology. Initial comparator studies on this pathway were mainly conducted on cells and tissues derived from mice and humans. This resulted in the discovery of anti PD-1 or anti-PD-L1 immune checkpoint therapy that is widely applied for the treatment of human cancers. The use of monoclonal antibodies directed against PD-1 or PD-L1 as therapeutic agents restores the anti-cancer immune response. In recent years, investigations on these molecules have been extended to canine cancers and confirm the expression of PD-1 and PD-L1 in several canine tumors. Whether immune checkpoint therapy may be a possible treatment option for those canine cancers remains to be revealed in future clinical trials.
- programmed death protein 1
- PD-1
- programmed death protein ligand 1
- PD-L1
- immuno-oncology
- cancer
1. Introduction
1.1 The immune checkpoint molecules PD-1 and PD-L1 1.1 The Immune Checkpoint Molecules PD-1 and PD-L1
The intensity of an immune reaction is the net result of the concurrent activation of one or several costimulatory vs coinhibitory or immune checkpoint molecules [1]. The latter include proteins such as programmed cell death protein 1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1) [1][2]. Physiological functions of the PD-1/PD-L1 pathway include inhibition of effector T cell activation [3] leading to induction of central and peripheral tolerance [2][3], maintenance of tissue homeostasis [3], and contribution to immune privilege status of certain organs and tissues [3]. The binding between PD-L1 expressed on antigen presenting cells (APCs) and PD-1 concurrently expressed on effector T cells, reduces the magnitude of immune cell activation [1][2]. During an acute immune reaction, transient upregulation of PD-1 on activated effector T cells occurs that terminates or at least attenuates the immune reaction [4]. PD-1 is also expressed on regulatory T cells (Tregs) [5]. Similarly, PD-L1 is also found on parenchymal cells, in particular those of immune privileged organs [3]. Pathological alterations of this pathway are observed in the setting of chronic infectious diseases, autoimmune reactions and cancer development and progression [3]. These are primarily associated with chronic antigenic stimulation, which leads to exhaustion of effector T cells and their continuous upregulation of PD-1 [3][6]. Further, PD-L1 and PD-1 can also be expressed by additional cell populations such as tumor cells [2][3][7].1.2 Comparative medicine1.2 Comparative Medicine
The basic principle of comparative medicine and the one-health one-medicine concept is that humans and companion animals share the same environment and develop similar diseases [8][9]. Therefore, clinically relevant information obtained from a particular disease in humans can be helpful for diagnosis or treatment of the same disease or a similar disease in a particular animal species and vice versa [9]. This applies not only to numerous infectious diseases, but also to occurrence of cancer in companion animals. Notably, annually more dogs than humans are newly diagnosed with malignant tumors, i.e., in the USA this encompasses about 4.2 million dogs compared to approximately 1.6 million human beings [9]. Human and canine cancers mostly develop spontaneously, and often are comparable in their clinical presentation, pathological findings and genomic alterations [9] . Since certain dog breeds have a predisposition to develop particular cancer types, they may represent possible animal models for those tumors in humans [9].
2. The PD-1/PD-L1 pPathway in the tumor mTumor Microenvironment
The tumor microenvironment (TME) is composed of different cell populations, extracellular matrix and different soluble mediators [7][10]. Cellular components encompass tumor cells, cancer associated fibroblasts (CAFs), vascular endothelial cells as well as a variety of immune and inflammatory cells [7][10]. The latter include components of the innate and adaptive immunity such as natural killer cells, macrophages, dendritic cells, neutrophils, myeloid derived suppressor cells, helper T cells, cytotoxic T cells, regulatory T cells and B cells [7][10]. Tumors with the presence of numerous immune cell infiltrates are referred to as “hot tumors”, whereas those associated with none or only a few immune cells represent “cold tumors”[10][11]. All cell types of the TME can express varying amounts of the immune checkpoint molecules PD-1 and/or PD-L1 [2][3][7][10] (Figure 1).
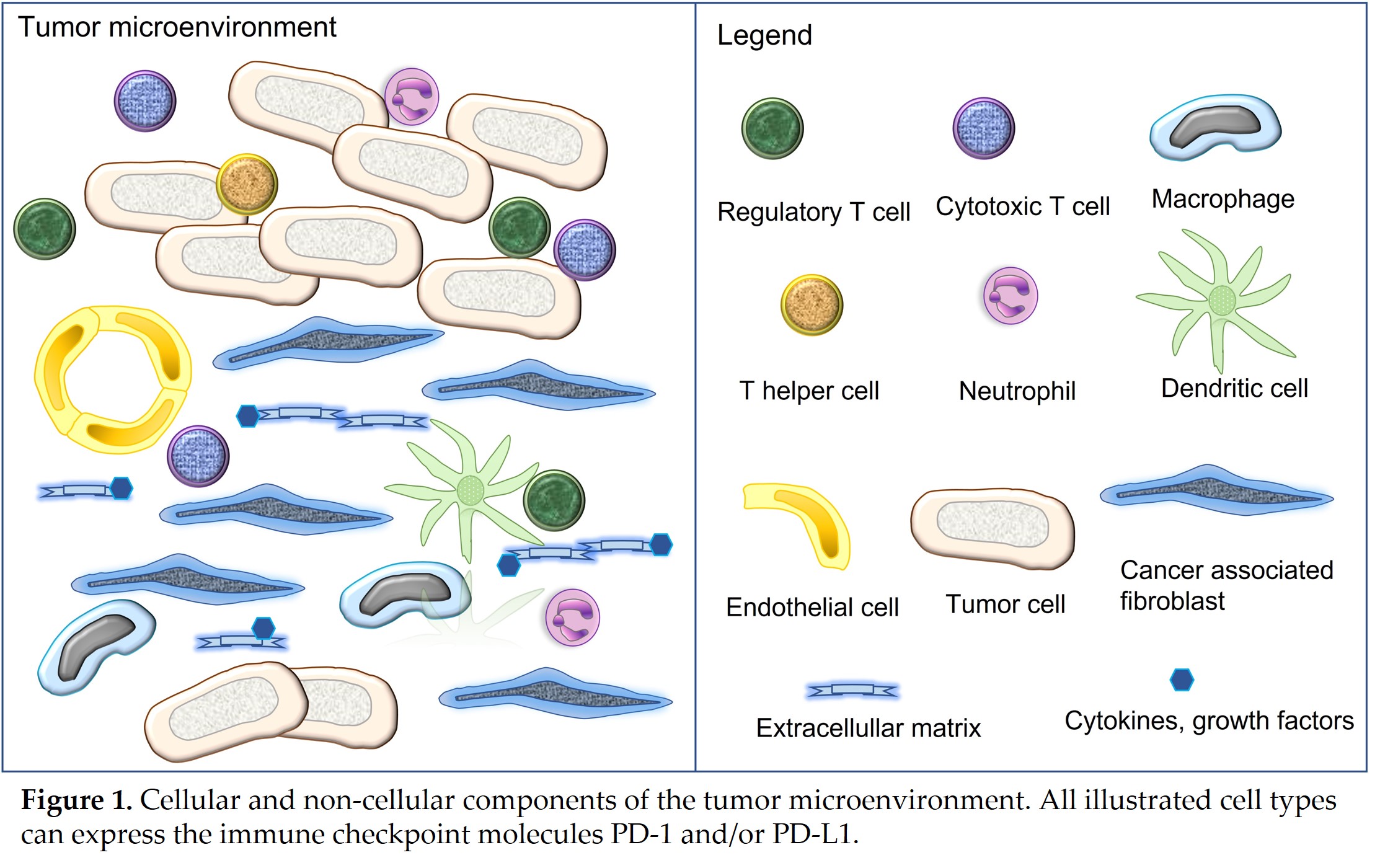
The presence of PD-L1 on cancer cells has been attributed to two main mechanisms, named as oncogenic and adaptive pathways [7][12]. Oncogenic PD-L1 expression is constitutive, it is caused by different types of mutations that upregulate PD-L1, and it usually involves all tumor cells [7][12]. Adaptive PD-L1 expression is induced by interferon γ and other proinflammatory mediators, affects tumor cells and immune cells, and is therefore a frequent finding in “hot tumors”[7][12]. The diagnostic hallmark of adaptive expression is multifocal PD-L1 immunostaining in those areas, where tumor cells and immune cells are located in close proximity [7][12]. Both pathways can also occur in combination [7][12]. Cancer cells of different tumor entities can also show PD-1 expression, which has been attributed to either genetic or epigenetic alterations or cytokine induction [7][13]. The same tumor cell can either harbor PD-L1 or PD-1 or show simultaneous expression of both molecules [7][13]. T cells in the TME are often exhausted with permanent upregulation of PD-1, this may also occur together with their PD-L1 expression [7].
2.1 The mMultiple fFacets of PD-L1 and PD-1 iInteraction in the tumor mTumor Microenvironment
Initially, the immunosuppressive action of the PD-1/PD-L1 pathway in the TME has been mainly attributed to the functional inhibition of effector T cells that is evoked by the binding between a PD-L1 bearing tumor cells and a PD-1 positive effector T cell [2][7][13]. Thereby, the effector T cell serves as target cell, in which the induced signal transduction pathways inhibit secretory activity, proliferation and survival [2][7]. The binding between PD-L1 and PD-1 molecules expressed on different cells is named as “trans” interaction, and the induction of inhibitory signalling in the PD-1 positive target cells is consistent with “forward” signalling [2].
Subsequent investigations, however, uncovered a high complexity of the PD-1/PD-L1 pathway, that includes the expression of PD-L1 and PD-1 on multiple cell populations [2][7], the interaction of both molecules not only on different cells, but also on the same cell (“cis” binding) [2][14], signal transduction through PD-1 (forward signalling) and PD-L1 (reverse signalling) [2][7], the presence of CD80 as additional PD-L1 ligand [2], and the extracellular release of PD-L1 and PD-1 [15].
2.1.2 Trans interaction of PD-L1 and PD-1 positive cells in the forward signaling mode2.1.2 Trans Interaction of PD-L1 and PD-1 Positive Cells in the Forward Signaling Mode
The TME does not only contain PD-L1 positive tumor cells, but also multiple other PD-L1 positive cell types such as CAFs [16], antigen presenting cells (APCs) [2][14], activated T cells [14] and also neutrophils [17][18]. All of these cells may bind to PD-1 on effector T cells and thus downregulate anti-cancer immune defenses exerted by type 1 T helper cells, cytotoxic T cells and natural killer cells [2][17][18]. Their interaction with a PD-1 bearing regulatory T cell [5] or myeloid derived supressor cell [19], however, will have the opposite effects, since it inhibits immunosuppressive functions exerted by these cells.
Similarly, in tumor cells with intrinsic PD-1 expression, its ligation by PD-L1 can also evoke tumor-type specific effects[7][13]. In melanomas, HCC and urinary bladder carcinomas, it stimulates tumor cell proliferation [7][13], whereas in some colon cancer cases and NSCLC it inhibits tumor growth [7][13].
Notably, signalling transduction through the PD-1 receptor not only modulates specific cellular functions, but it can even lead to cellular trans-differentiation, e.g., transformation of a cytotoxic T cell into a regulatory T cell [2].
2.1.3 Cis interaction of PD-L1 and PD-1 positive cells 2.1.3 Cis Interaction of PD-L1 and PD-1 Positive Cells
PD-L1 and PD1 that are co-expressed on the same cell can interact with their respective binding partner on the same cell (“cis”) or on another cell (“trans”). The “cis” interaction reduces the number of immune checkpoint molecules available for “trans” interaction [2][14]. It cannot be ruled out, however, that “cis” interaction may not only neutralize these molecules, but also trigger signal transduction events [14].
2.1.4 Additional binding partner for PD-L12.1.4 Additional Binding Partner for PD-L1
CD80 represents another binding partner for PD-L1 [2]. The binding between these two molecules can be in “cis” or “trans” and reduces inhibitory signalling through PD-1 [2].
2.1.5 Reverse signalling mode 2.1.5 Reverse Signalling Mode
The binding between PD-L1 and PD-1 may not only trigger signal transduction events in the PD-1 expressing cell, but also in the PD-L1 positive cell [2][7][13]. For example, PD-1 positive tumor cells are reported to inhibit cytotoxicty of PD-L1 expressing neutrophils [20], and PD-L1 positive M2 macrophages may trigger trans-differentiation of type 1 helper T cells to type 17 helper T cells [2].
2.1.6 Extracellular PD-L1 and PD-1 molecules2.1.6 Extracellular PD-L1 and PD-1 Molecules
Different cell populations can shed PD-L1 and PD-1 in the extracellular space and serum [15][21]. Those PD-L1 molecules may induce immunosuppressive effects by binding to cell-associated PD-1 [21]. In comparison, extracellular PD-1 has been reported to mediate endocytosis of cell-bound PD-L1, which would restore immune cell activation [21]. In addition, binding between soluble PD-1 and soluble PD-L1 molecules could prevent their interaction with cell-associated partner molecules [21].
2.2 The cClinical iImportance of PD-L1 as pPredictve bBiomarker
The immunohistochemical detection of PD-L1 expression in the TME serves as biomarker to decide on a patient’s eligibility for immune checkpoint therapy that uses anti-PD-1 or anti-PD-L1 antibodies [7][12][22]. These antibodies block the interaction of between PD-1 and PD-L1 and restore the anti-cancer immune response [2][7][12][22].
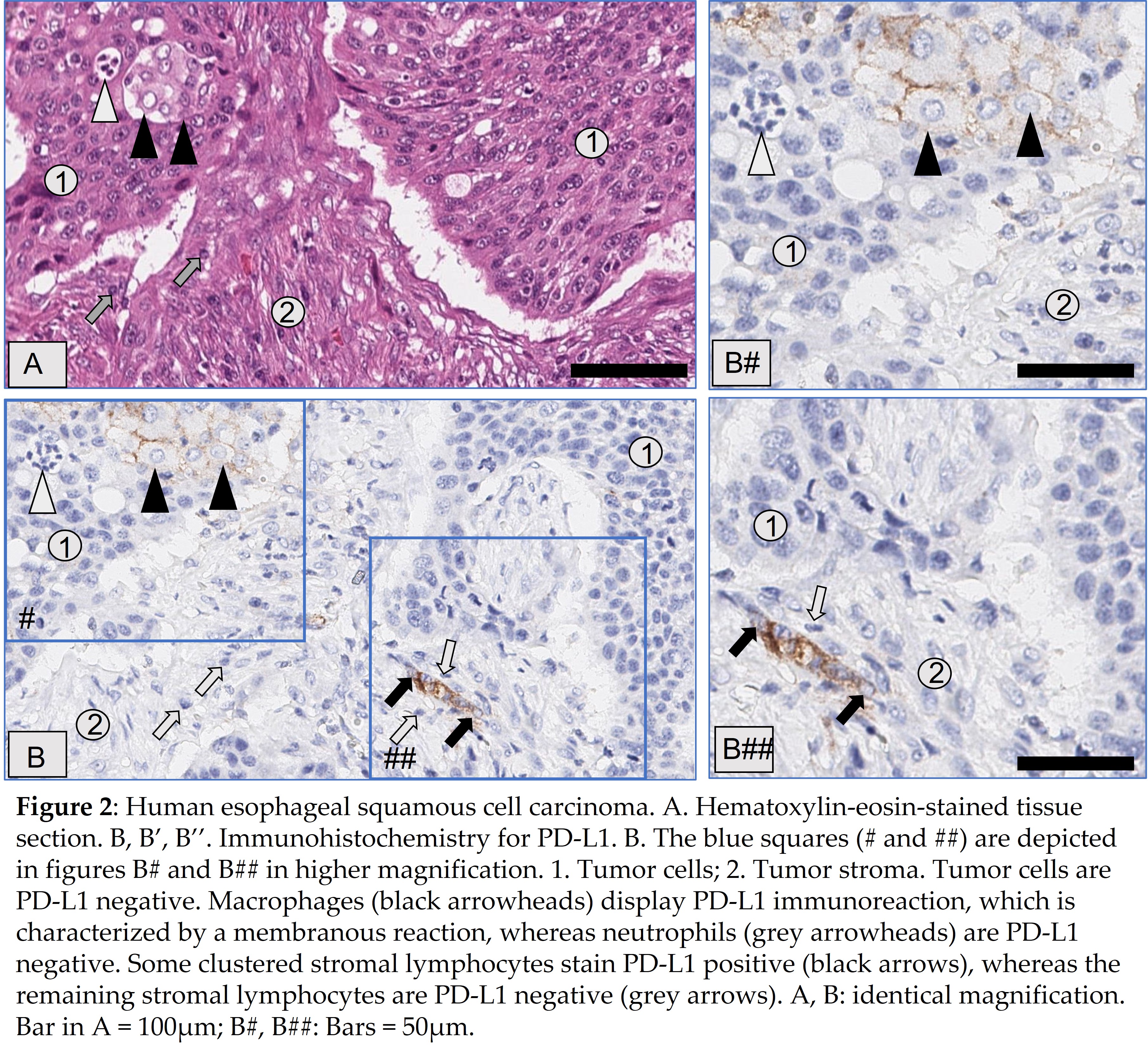
To detect PD-L1 expression on tumor and immune cells, different diagnostic anti-PD-L1 antibodies are available that are used in combination with associated staining platforms [Jöhrensen and Rüschoff, 2021]. Results are interpreted under application of the relevant score(s), i.e., tumor proportion score (TPS), combined proportion score (CPS), imunoscore (IC), and their respective cut-off values, which differ between cancer-types and first- or second-line therapy [22] (Figure 2).
3. Biomarker research in the context of comparative immuno-oncology
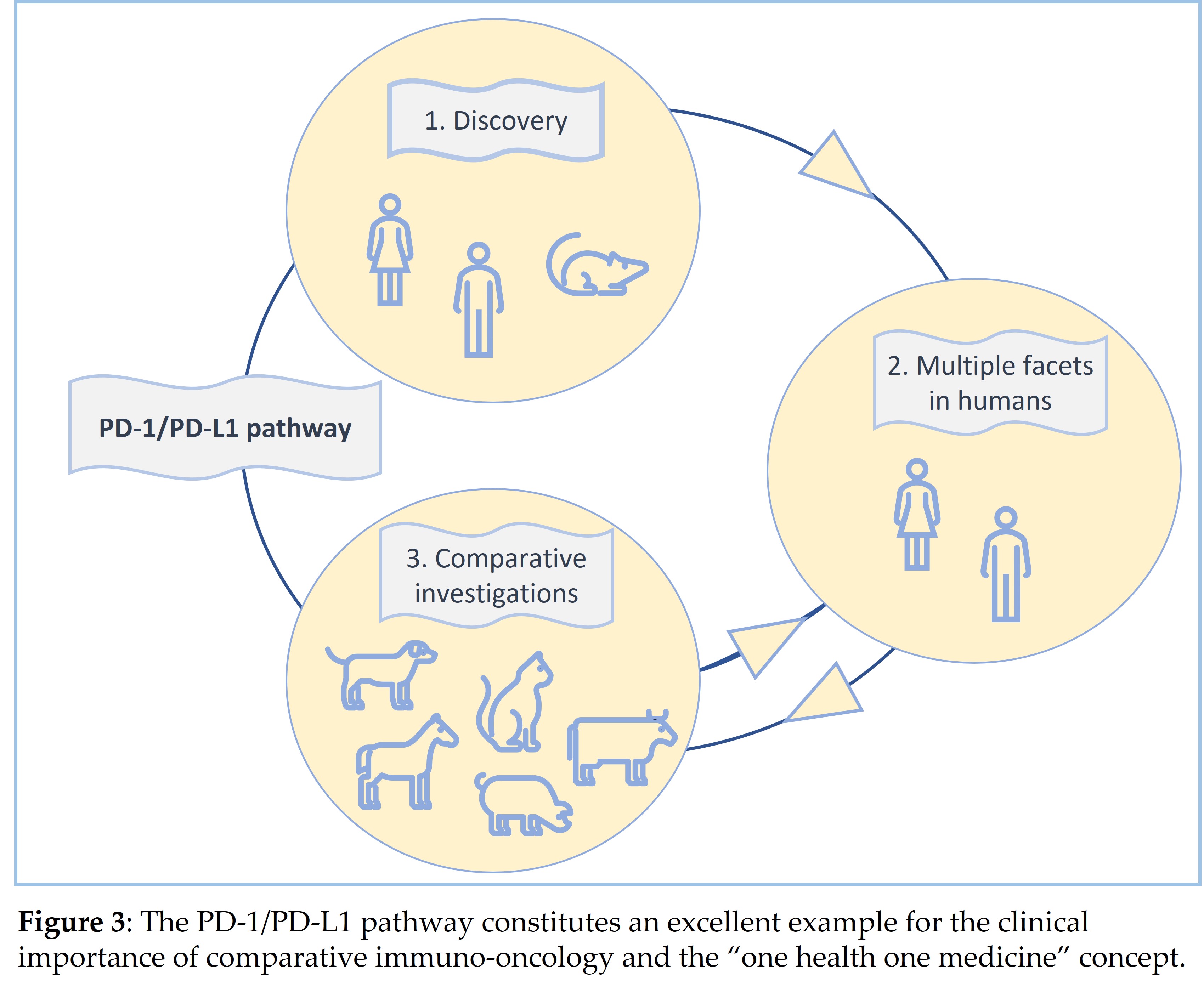
The PD-1/PD-L1 pathway is an excellent paradigm for the clinical importance of comparative immuno-oncology and the “one health one medicine” concept. During the discovery phase of the PD-L1/PD-1 pathway, most studies were performed on murine and human cells and tissues [23][24][25][26][27][28]. The subsequent investigations focused on the pathophysiological role of the PD-1/PD-L1 pathway in human beings including its physiological functions in different tissues as well as its contribution to chronic infectious diseases, autoimmune reactions as well as tumorigenesis and cancer progression [2][3][28][29].
This has also led to the discovery of immune checkpoint therapy, for which James Allison and Tasuku Honjo received the Nobel prize Medicine in 2018 [30][31]. Nowadays, immune checkpoint therapy is widely used for the treatment of numerous human cancer entities [7][12][31].
Subsequent research, however, has led to the identification of an increased complexity of the PD-1/PD-L1 pathway in the TME, which not only modulates the biological behavior, but likely also influences the success of immune checkpoint therapy.
In recent years, studies on the PD-1/PD-L1 pathway have been expanded to cells and tissues of farm and companion animals. The aim of these comparative investigations has been to characterize the functions of this pathway also in different animal species and to compare observed findings with those known from humans and mice. Ultimate goals of these studies have been to develop overlapping diagnostic and treatment options for humans and different animal species and to identify animal models for human cancer and additional human diseases involving the PD-1/PD-L1 pathway [28].
Most of the above investigations have been performed on canine cancers [32][33][34][35][36][37] due to the high frequency of neoplastic disease in dogs and remarkable similarities between human and canine tumors [9]. This has led even to the production of a chimeric anti-canine PD-L1 monoclonal antibody, which has already been used in a pilot clinical trial involving nine dogs with malignant tumors [34]. Two of the treated dogs showed tumor regression as response to treatment with this antibody [34] (Figure 3).
4. Conclusions
4.1 The immune checkpoint molecules PD-1 and PD-L1
4.1 The Immune Checkpoint Molecules PD-1 and PD-L1
The described dynamic regulations of the expression of the immune checkpoint molecules PD-1 and PD-L1 on tumor cells, CAF and multiple immune cell subsets together with the high plasticity of immune cells have the capacity to not only influence the biological behaviour of the tumor, but also the tumor’s response to immune checkpoint therapy. Therefore, immunohistochemical examination of a tumor sample for expression of PD-L1 has to be regarded as snapshot of time [38] that is likely to be influenced by changes in the TME, e.g., those caused by hypoxia [5] or radiochemotherapy
4.2 Comparative medicine4.2 Comparative Medicine
The PD-L1/PD-1 pathway represents an excellent example for biomarker research in the context of comparative immuno-oncology. Results of an initial clinical pilot trials in dogs with cancer have already been published [34]. In addition, transgenic pigs expressing human PD-1 have been produced [39]. Additional comparative investigations into predictive cancer biomarkers are ongoing, an excellent example is the detection of mismatch repair deficiency (dMMR) [40]. Human tumors with dMMR usually show a favorable response to immune checkpoint therapy [41]. Interestingly dMMR has been recently also diagnosed in different canine tumors [40].
References
- Chen L, Flies DB; Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013, 13(4), 227-242, doi:10.1038/nri3405.
- Wang Q, Bardhan K, Boussiotis VA, Patsoukis N; The PD-1 interactome. Adv Biol (Weinh). 2021, 5(9), e2100758, doi:10.1002/adbi.202100758.
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X; The diverse function of PD-1/PD-L pathway beyond cancer. Front Immunol. 2019, 10, 2298, doi:0.3389/fimmu.2019.02298.
- Jubel JM, Barbati ZR, Burger C, Wirtz DC, Schildberg FA; The role of PD-1 in acute and chronic infection. Front Immunol. 2020, 11, 487, doi:10.3389/fimmu.2020.00487.
- Tay C, Qian Y, Sakaguchi S; Hyper-progressive disease: The potential role and consequences of T-regulatory cells foiling anti-PD-1 cancer immunotherapy. Cancers (Basel). 2013, 13(1), 48, 10.3390/cancers13010048.
- Dolina JS; Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S; CD8+ T cell exhaustion in cancer. Front Immunol. 2021, 12, 715234, doi:10.3389/fimmu.2021.715234.
- Hudson K; Cross N, Jordan-Mahy N, Leyland R; The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: Implications for immunotherapy treatment. Front Immunol. 2020, 11, 568931, doi:10.3389/fimmu.2020.568931.
- Sundberg JP, Schofield PN.; One medicine, one pathology, and the one health concept. J Am Vet Med Assoc. 2009, 234(12), 1530-1531, doi:10.2460/javma.234.12.1530.
- Schiffman JD, Breen M; Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. 2015, 370(1673), 20140231, doi:10.1098/rstb.2014.0231.
- Bożyk A, Wojas-Krawczyk K, Krawczyk P, Milanowski J; Tumor microenvironment-A short review of cellular and interaction diversity. Biology (Basel) 2022, 11(6), 929, doi:10.3390/biology11060929.
- Galon J, Bruni D; Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019, 18, 197-218, doi:10.1038/s41573-018-0007-y.
- Ribas A, Hu-Lieskovan S; What does PD-L1 positive or negative mean?. Exp Med. 2016, 213(3), 2835-2840, doi:10.1084/jem.20161462.
- Yao H, Wang H, Li C, Fang JY, Xu J; Cancer cell-intrinsic PD-1 and implications in combinatorial immunotherapy. Front Immunol. 2018, 9, 1774, doi:10.3389/fimmu.2018.01774.
- Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E; Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep. 2018, 24(2), 379-390, doi:10.1016/j.celrep.2018.06.054.
- Vautrot V, Bentayeb H, Causse S, Garrido C, Gobbo J; Tumor-derived exosomes: hidden players in PD-1/PD-L1 resistance. Cancers (Basel) 2021, 13(18), 4537, doi:10.3390/cancers13184537.
- Gorchs L, Fernández Moro C, Bankhead P, Kern KP, Sadeak I, Meng Q, Rangelova E, Kaipe H; Human pancreatic carcinoma-associated fibroblasts promote expression of co-inhibitory markers on CD4+ and CD8+ T-cells. Front Immunol. 2019, 10, 847, doi:10.3389/fimmu.2019.00847.
- He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L, Chen H, Zeng H.; Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular car-cinoma. J Exp Clin Cancer Res. 2015, 34, 141, doi:10.1186/s13046-015-0256-0.
- Tang D, Zhang D, Heng Y, Zhu XK, Lin HQ, Zhou J, Tao L, Lu LM; Tumor-infiltrating PD-L1+ neutrophils induced by GM-CSF suppress T cell function in laryngeal squamous cell carcinoma and predict unfavorable prognosis. J Inflamm Res. 2022, 15, 1079-1097, doi:10.2147/JIR.S347777.
- Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, Pal R, Yuan M, Asara J, Patsoukis N, Boussiotis VA; Tumor-infiltrating PD-L1+ neutrophils induced by GM-CSF suppress T cell function in laryngeal squamous cell carcinoma and predict unfavorable prognosis. Sci Immunol. 2022, 15, 1079-1097, doi:10.1126/sciimmunol.aay1863.
- Yajuk O, Baron M, Toker S, Zelter T, Fainsod-Levi T, Granot Z; The PD-L1/PD-1 axis blocks neutrophil cytotoxicity in cancer. Cells. 2021, 10(6), 1510, doi:10.3390/cells10061510.
- Qiu Y, Yang Y, Yang R. Liu C, Hsu JM, Jiang Z, et al; Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene 2021, 40, 4992–5001, doi:10.1038/s41388-021-01896-1.
- Jöhrens K, Rüschoff J; The challenge to the pathologist of PD-L1 expression in tumor cells of non-small-cell lung cancer-an overview. Curr Oncol. 2021, 28(6), 5227-5239, doi:10.3390/curroncol28060437.
- Ishida Y, Agata Y, Shibahara K, Honjo T; Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992, 11(11), 3887-3895, doi:10.1006/geno.1994.1562.
- Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T; Structure, and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 1994, 23(3), 704-706, doi:10.1006/geno.1994.1562.
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T; Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996, 8(5), 765-772, doi:10.1093/intimm/8.5.765.
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T; Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151, doi:10.1016/s1074-7613(00)80089-8.
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al; Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000, 192(7), 1027-1034, doi:10.1084/jem.192.7.1027.
- Schöniger S, Jasani B; The PD-1/PD-L1 pathway: A perspective on comparative immuno-oncology. Animals (Basel). 2022, 12(19), 2661, doi:10.3390/ani12192661.
- Sharpe A, Pauken K; The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018, 18, 153-167.
- Ledford H, Else H, Warren M; Cancer immunologists scoop medicine Nobel prize. Nature 2018, 18, 153-167, doi: 10.1038/d41586-018-06751-0.
- Huang PW, Chang JW; Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed J. 2019, 42(5), 299-306, doi: 10.1016/j.bj.2019.09.002.
- Maekawa N, Konnai S, Ikebuchi R, Okagawa T, Adachi M, Takagi S, et al; Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade. PLoS One 2014, 9(6), e98415, doi:10.1371/journal.pone098415.
- Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al; Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE. 2016, 11, e0157176, doi:10.1371/journal.pone.0157176.
- Maekawa N, Konnai S, Takagi S, Kagawa Y, Okagawa T, Nishimori A, et al.; A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undiffer-entiated sarcoma. Sci Rep. 2017, 7(1), 8951, doi:10.1038/s41598-017-09444-2.
- Hartley G, Faulhaber E, Caldwell A, Coy J, Kurihara J, Guth A, et al; Immune regulation of canine tumour and macrophage PD-L1 expression. Vet Comp Oncol. 2017, 15(2), 534-549, doi:10.1111/vco.12197.
- Aresu L, Marconato L, Martini V, Fanelli A, Licenziato L, Foiani G, et al; Prognostic value of PD-L1, PD-1 and CD8A in canine diffuse large B-Cell lymphoma detected by RNAscope. Vet Sci. 2021, 8(7), 120, doi:10.3390/vetsci8070120.
- Pinard, C.J.; Hocker, S.E.; Poon, A.C.; Inkol, J.M.; Matsuyama, A.; Wood, R.D.; et al.; Evaluation of PD-1 and PD-L1 expression in canine urothelial carcinoma cell lines. . Vet Immunol Immunopathol 2022, 243, 110367, doi:10.1016/j.vetimm.2021.110367. .
- Feng Z, Bethmann D, Kappler M, Ballesteros-Merino C, Eckert A, Bell RB, et al.; Multiparametric immune profiling in HPV- oral squamous cell cancer. JCI Insight 2017, 2(14), e93652, doi:10.1172/jci.insight.93652.
- Buermann A, Petkov S, Petersen B, Hein R, Lucas-Hahn A, Baars W, et al; Pigs expressing the human inhibitory ligand PD-L1 (CD 274) provide a new source of xenogeneic cells and tissues with low im-munogenic properties. Xenotransplantation. 2018, 25(5), e12387, doi:10.1111/xen.12387.
- Inanaga S, Igase M, Sakai Y, Tanabe M, Shimonohara N, Itamoto K, et al; Mismatch repair deficiency in canine neoplasms. Vet Pathol 2021, 58(6), 1058-1063, doi: 10.1177/03009858211022704.
- Schöniger S, Rüschoff J; Mismatch repair deficiency and microsatellite instability. Encyclopedia 2022, 2, 1559-1576, doi:10.3390/encyclopedia2030106.
