Gastric cancer (GC) is the fifth most commonly diagnosed cancer worldwide and the fourth most common cause of cancer death. As GC is often diagnosed at an advanced stage, mortality remains very high. GC shows both genetic and environmental risk factors, with Helicobacter pylori (H. pylori) infection being the most well-described risk factor leading to GC. Germline genetic alterations are also involved in 1–3% of cases. Due to the poor survival rates of GC, immunotherapy has been widely explored as a potential treatment. Both active and passive immunotherapies have been examined. Active immunotherapies involve using a patient’s own immune system to treat the disease whereas passive immunotherapies rely on exogenous agents administered to patients such as antibodies in order to treat the tumour. The great efficacy observed in melanoma has propelled immunotherapies to be explored in a variety of other tumours, particularly breast, prostate, and lung cancer].
- gastric cancer
- immunotherapy
- PD-L1
- H. pylori
- EBV
- immune microenvironment
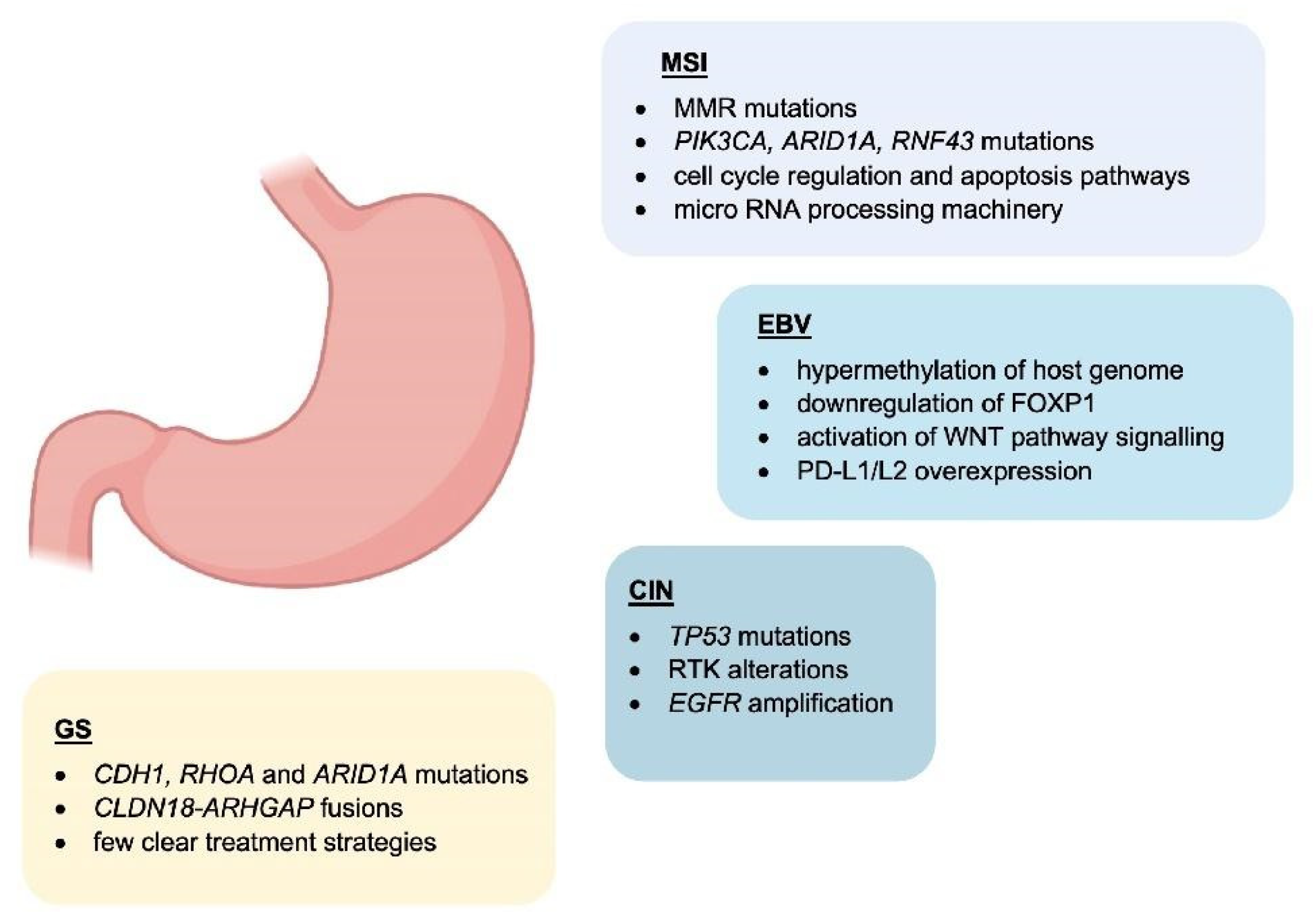
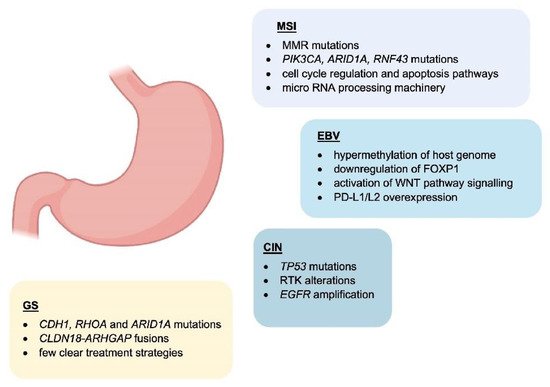
1. Molecular Classification
2. The Role of Tumour-Infiltrating Immune Cells in the Gastric Cancer Microenvironment
3. Role of Chronic Infection with Helicobacter pylori and the Association with an Immunosuppressive Microenvironment
Helicobacter pylori (H. pylori) is a facultative, spiral-shaped, Gram-negative bacterium that selectively colonises the gastrointestinal mucosa [32][33][34][45,46,47]. Four decades after its discovery in 1982, H. pylori represents a well-established risk factor for GC, and it is recognised as a type I carcinogen by the International Agency for Research on Cancer (IARC), with 90% of non-cardia gastric cases attributable to this bacterium [34][35][36][37][47,48,49,50].3.1. Contribution of H. pylori Virulence Factors to Chronic Inflammation
During H. pylori infection, stimulation of several inflammatory signals is key to the establishment of an inflammatory environment in the gastric epithelium. This marks an important step towards initiation of a more complex inflammatory and immune response which can eventually culminate in the development of peptic ulceration and gastric malignancies. A range of genes expressed by H. pylori are involved in the infection and remodelling process of the microenvironment [32][38][39][45,54,55]. These include ureases that confer this pathogen the ability to colonise and neutralise the highly acidic environment found within the stomach by converting urea into ammonia, therefore establishing the optimal pH conditions for its growth [38][54]. The subsequent increase in pH contributes to alter the viscosity of gastric mucus facilitating H. pylori diffusion through the mucosal barrier and allowing the pathogen to gain access to the underlying epithelial cells [40][56]. This event is crucial for the gastric epithelium colonisation process, which is the basis of the inflammatory reaction induced by H. pylori. Moreover, urease could contribute to gastric carcinogenesis by producing reactive oxygen species and activating the lipoxygenase pathway, resulting in differentiation of endothelial cells [41][42][57,58]. H. pylori flagella favour the colonisation of the gastrointestinal mucosa and contribute to bacterial motility [43][59]. FlaA is one of the main structural proteins of the H. pylori flagellum which can evade the host immune response, as it can escape recognition by the Toll-like receptor 5 (TLR5), a member of the Toll-like receptor family that normally recognises most bacterial flagellins [43][44][59,60]. As flagellins are critical for persistent H. pylori colonisation, and given that H. pylori colonisation is the basis of inflammation, flagella can be considered responsible for both inflammation and immune evasion [33][43][46,59]. Other players can also act in a cascade of events inducing damage to the gastric mucosa and host inflammatory response. Amongst these, there is the cytotoxin-associated gene CagA which is part of the cag pathogenicity island (cag PAI)—locus of approximately 40 kb containing 31 genes, the majority of which encode for the cag secretion system (T4SS) [33][44][46,60]. Importantly, cag Pal+ H. pylori strains increase the risk of gastritis, atrophic gastritis, and GC than strains lacking the cag island [45][61]. CagA modulates the host cell signalling both in a phosphorylation-dependent and independent manner. Phosphorylation of CagA has been reported to induce sustained activation of the ERK1/2 MAP kinase and NF-kB signalling pathways, and disruption of epithelial cell tight junctions with damage to the gastric mucosa [41][57]. Activation of proinflammatory responses, mostly through IL-8 pro-inflammatory cytokine production instead, appears to be independent of CagA phosphorylation [33][44][46][46,51,60].3.2. Host Immune Response to H. pylori Infection
4.2. Host Immune Response to H. pylori Infection
During H. pylori infection, both innate and acquired immune responses are intensely stimulated. Despite the strong immune responses, H. pylori has the remarkable ability to persist for a very long time in the gastric mucosa, actively modulating and evading the host response to establish an immunosuppressive environment that maintains chronic infection [47][52]. Most of the factors mentioned above are thought to intensify local inflammation with consequent infiltration of inflammatory cells to the gastric mucosa. Innate host defence mechanisms are triggered as a first line of defence and are crucial to increase risk of gastric carcinogenesis and severity of the disease [44][60]. The innate immune response includes the nucleotide-binding oligomerisation domain protein 1 (Nod1) [48][64]. This is a pattern recognition receptor (PRR) responsible for the recognition of H. pylori peptidoglycan components secreted by the cag secretion system, and activation of NF-kB-dependent proinflammatory responses. The most studied PRRs are the Toll-like receptors (TLRs), expressed on epithelial and innate immune cells, which interact with diverse H. pylori antigens (including lipoteichoic acid, lipoproteins, lipopolysaccharide, and flagellin) and initiate the adaptive immune responses [49][65]. Amongst the main players of the innate response to H. pylori, there are macrophages which, along with monocytes and DCs, are responsible for the recruitment of lymphocytes and the stimulation of T-helper (Th) cell-specific responses by releasing factors such as IL-12. In particular, stimulation of Th1 cells is predominant, and it is central in the T-cell response to H. pylori, as it produces cytokines such as IFN-γ and leads to pro-inflammatory cytokine release, for example TNFα, and interleukins [50][67] (Figure 2).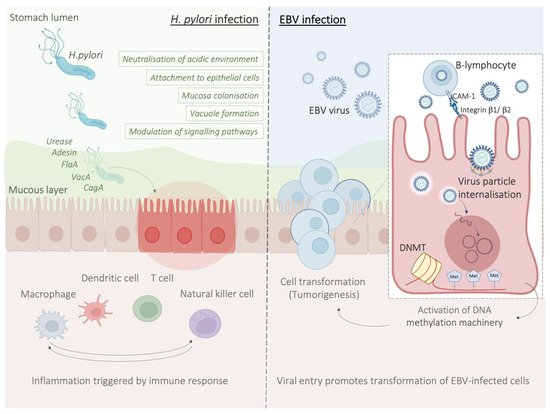
3.3. H. pylori Infection Promotes an Immunosuppressive Tumour Microenvironment
4.3. H. pylori Infection Promotes an Immunosuppressive Tumour Microenvironment
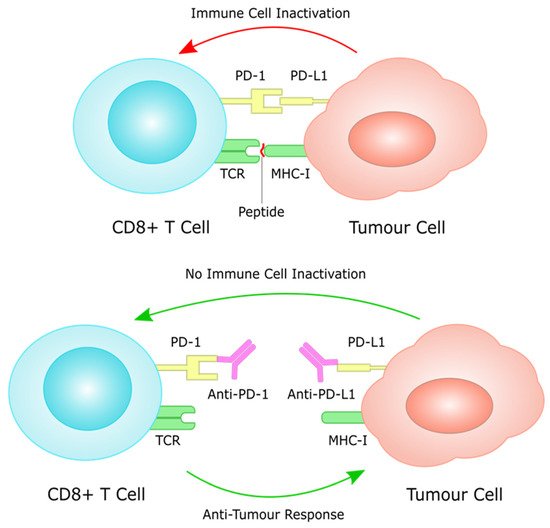
As discussed above, the establishment of H. pylori-mediated chronic infection has been shown to have profound implications on the host immune system and its functions. In fact, by exerting immunomodulatory effects on a variety of immune cells (through negative modulation of Th1 and Th17 cells, macrophages and dendritic cells, and promotion of Treg cells activity) H. pylori is thought to be responsible for the creation of an immunosuppressive environment [51][52][70,71]. Under normal conditions, the immune system retains the ability to generate tumour-specific immune responses to thwart cancer formation and progression, a concept known as anti-tumour immunity [53][72]. Such anti-tumour immunity can be boosted by cancer immunotherapies, for instance by using immune checkpoint inhibitors (ICIs) that block the natural function of immune checkpoints and prevent attenuation of immune cell activation [52][71]. These include inhibitors of the cytotoxic T-lymphocyte antigen 4 (CTLA-4), or the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) [54][73].4. Molecular Mechanisms Underlying the Pathology of Microsatellite Instable Gastric Cancer
Genomic instability is one of the major hallmarks of cancer development [55][84]. It is believed to be one of the initial steps of gastric carcinogenesis and can be found in all different histological subtypes of GC [56][85]. Microsatellites (MS) are repetitive and specific DNA sequences characterized by a high mutation rate [57][58][86,87]. Microsatellite instability (MSI) is a hyper-mutable phenotype caused by a non-functional DNA mismatch repair (MMR) machinery at MS sites. During DNA replication, the insertion or deletions of nucleotides in MS regions because of germline mutations or epigenetic silencing cause malfunction of MMR system [59][60][88,89]. The MMR system includes several proteins: human MutL homolog 1 (hMLH1), human MutL homolog 3 (hMLH3), human MutS homolog 2 (hMSH2), human MutS homolog 3 (hMSH3), human MutS homolog 6 (hMSH6), human post meiotic segregation increased 1 (hPMS1), and human post meiotic segregation increased 2 (hPMS2) [58][87]. During DNA replication, hMSH2/hMSH6 and hMSH2/hMSH3 complexes are responsible for detecting and binding small DNA mismatch errors while the excision and re-synthesis of the corrected DNA bases in the mismatch site is detected by the heterodimeric complex hMLH1/hPMS2. Defects in one or more MMR machinery elements determine the unsuccessful repair of the DNA [58][87]. Different processes including promoter methylation, chromosomic rearrangements that lead to loss of heterozygosity or mutations in the coding region are responsible for the inactivation of MMR proteins [61][62][90,91]. The main cause of MMR deficiency in both sporadic and familial MSI GCs is represented by the hypermethylation of hMLH1 promoter [63][64][92,93]. Conversely, mutations of hMLH1 and hMSH2 are relatively rare (15% and 12%, respectively) [65][94]. Some reports showed that MSI represents an early molecular event during gastric carcinogenesis [66][67][95,96]. However, Ling et al. reported that the promoter methylation of hMLH1 represents a later event during the natural process of tumour growth and the time-dependent acquisition of MSI may be due to the hMLH1 silencing [68][97]. Both sporadic GC and Lynch syndrome (LS) show MSI [62][69][91,98]. LS is mainly caused by autosomal dominant mutations affecting hMLH1 and hMSH2 and less frequently hPMS2 and hMSH6 [62][91]. Moreover, the epigenetic silencing of hMSH2 by a constitutional 3′ end deletion of EPCAM can also cause LS [70][71][99,100]. Patients affected by LS show an increased predisposition to develop GC at a younger age (11.3-fold in the 30s and 5.5-fold in the 40s) [61][62][90,91].5. EBV-Related Gastric Cancer
Epstein–Barr virus (EBV) emerged as an important virulence factor for nasopharyngeal carcinoma. EBV infection also causes the development of T-cell lymphoma and EBV-associated GC (EBVaGC) [72][73][106,107]. Immunotherapy drug treatments were successful against EBV-positive and MSI GCs [74][108]. Once EBV infects the human body, this does not immediately produce GC and EBV-positive GC is not characterized by any evident clinical manifestations [75][76][109,110]. Two theories have been reported about the mechanism of EBV infection. According to the first theory, EBV produces the infection of B-lymphocytes and oral epithelial cells [77][111]. In particular, since EBV is present in the saliva, this causes the infection of the epithelial cells [77][111]. The second theory reports EBV reactivation in B-lymphocytes in the stomach and its subsequent release to cause the infection of epithelial cells [77][111]. EBV infection of lymphocytes results in the interaction of these cells with epithelial cells [78][112]. This interaction is mediated by integrin β-1/β-2 and the translocation of intracellular adhesion molecule-1 to the cell surface (ICAM-1) produce cell-to cell contact [78][112]. The virus particles are then internalised by recipient cells through clathrin-mediated endocytosis [78][112]. EBV-particles inside the host cell nucleus are characterized by a naked, linear DNA genomes and a specific protein capsid protect them [79][113]. The exposed DNA linear genome is then circularised into a functional chromosome [79][113]. After circularisation, the chromatinised viral DNA protects it from DNA damage and provides accurate regulation of gene expression [79][113] (Figure 2). EBV genome is characterised by widely methylated CpG motifs enabling it to establish latent infection [80][114]. The two types of infection caused by EBV are the lytic and the latent form [80][114]. The latent infection is the one preferred by EBV and during the long incubation period, EBV causes the methylation of the host DNA and the expansion of GC [79][80][113,114]. Latent EBV proteins such as EBERs, BARF-0, EBNA-1, and LMP2A downregulate the miR-200 family causing a reduction in E-cadherin expression [81][115]. This mechanism is mediated by the upregulation of the E-cadherin repressors, ZEB1 and ZEB2 [81][115]. Tumour progression involves the loss of cell-to-cell adhesion and this event is also an important step in the carcinogenesis of EBVaGC [81][115]. EBV is the first human virus expressing many microRNAs [82][116]. The EBV miRNA BART11 has been shown to downregulate forkhead box protein P1 (FOXP1) transcription factor [83][117]. FOXP1 downregulation activates the epithelial-mesenchymal transition involving the gastric tumour cells or affecting the tumour microenvironment [83][117]. This, in turn, accelerates cancer invasion and metastasis, thereby affecting the survival and prognosis of patients [83][117].6. Potential Role and Relevance of POLE/D Mutations in Gastric Cancer
The immune checkpoint inhibitor (ICI) biomarkers approved by the US Food and Drug Administration (FDA) in the treatment of certain cancers are the use of PD-L1 expression, microsatellite instability (MSI)-H/deficient mismatch repair (dMMR) and tumoural mutation burden (TMB) [84][85][86][123,124,125]. Since some responses seen with ICIs do not fully correlate with any of the biomarkers above, other potential predictive biomarkers of response to ICI can exist in GC [87][88][126,127]. POLE and POLD1 genes encode for the DNA polymerases ε and δ [88][89][127,128]. TMB and clinical benefits of immunotherapy have been associated with mutations in POLE and POLD1 in different cancer types [90][91][129,130].7. HER2 Overexpression in Gastric Cancer
Human epidermal growth factor receptor 2 (HER2) is a receptor tyrosine kinase proto-oncogene that is increasingly understood to be overexpressed in GC. Different studies have found HER2 overexpression to occur in between 4.4% and 53.4% of GCs, with an average of 17.9% [92][132]. Prognostically, HER2 appears to be correlated with poorer survival and increased recurrence in GC [93][94][133,134]. HER2 targeting through the monoclonal antibody trastuzumab together with chemotherapy has in fact been shown to increase survival in HER2+ GC patients; however, the effect is small with a clinical trial only finding survival increasing from 11.1 months to 13.8 months [95][135]. During treatment, the tumour microenvironment contributes to tumourigenesis and proliferation but is also associated with immune cell infiltration and treatment efficacy [96][136]. Suh et al. reported that the HER2 pathway modulates the tumour microenvironment and this is correlated with tumour pathological characteristics and patient survival [97][137].8. Immunotherapy in the Clinical Management of Gastric Cancer
Over the last few years, immunotherapy has been actively incorporated as part of first and later lines of systemic treatment for advanced gastric cancer. PD-1 inhibitors have been shown to significantly improve efficacy in several large phase III trials when added to platinum-based chemotherapy, which has for many years been the standard of care in the first line setting for metastatic GC (Figure 3).