You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Jonaid Ahmad Malik.
Epithelial-mesenchymal transition (EMT) is initiated by EMT activating transcription factors (EMT-TFs), including SNAIL (SNAI1) and SLUG (SNAI2), the basic helix–loop–helix factors TWIST1 and TWIST2. As proven for SNAIL, TWIST, Zinc figure E-box binding homeobox 1 (ZEB1), and Zinc figure E-box binding homeobox 2 (ZEB2), these features can repress epithelial genes like the E-cadherin-producing CDH1 by binding to E-Box in their cognate promoter regions.
- epithelial-mesenchymal transition
- cancer EMT
- natural chemical entities
1. Artemisinin (ATM)
ATM is a sesquiterpene lactone isolated from sweet, warm wood, Artemisia annua. It is an antimalarial agent to treat multidrug-resistant falciparum malaria strains, mediated by producing organic peroxides [59][1]. ATM also has potent anti-cancer activity against CRC, BC, gastric cancer (GC), and cervix cancer (CC). Its anti-carcinogenic action is similar to antimalarial action in that free iron cleaves its endoperoxide bridge, releasing free radicals that cause cytotoxicity. ATM’s low toxicity and high specificity for cancer cells led to its development as an anti-cancer molecule. Dihydroartemisinic acid (DHA), an ATM derivative, reduced inflammation in a rat arthritis model by downregulating Interleukin-6 (IL-6). Additionally, DHA has anti-cancer properties. It can induce apoptosis in leukemic cells via noxa-mediated mechanisms [60][2].
Moreover, it inhibits GC cell invasion, migration, and proliferation by inhibiting the activation of phosphoinositide 3-kinase/protein kinase B and SNAIL. According to Sun et al., DHA’s anti-inflammatory and anti-cancer activities are mediated via microRNAs (miRNAs). DHA, for example, inhibits inflammation in vascular smooth muscle cells by regulating the miR-376b-3p/KLF pathway. The Jumonji and AT-rich interactive domain2 (ARID-2)/miR-7/miR-34a pathway inhibit prostate cancer cells by downregulating AXL tyrosine. In laryngeal cancer, miRNAs, in particular, play a function in EMT. For example, miR-217 inhibits EMT while miR-10b promotes it. miR-130b-3p is a tumor suppressor because it inhibits laryngeal cancer development, angiogenesis, migration, and invasion [61][3]. FoxM1, a member of the conserved forkhead box transcription factor family, is involved in cell cycle regulation, DNA damage repair, and apoptosis and has been associated with the development of breast, pancreas, and liver carcinomas. Nandi et al. hypothesized that FoxM1 was a critical inhibitory target of ATM in hepatocellular carcinoma (HCC) and that FoxM1 may play a role in the cell cycle triggered by DHA ATM-inhibited HCC cell survival and proliferation by attenuating FoxM1 and its transcription targets and interfering with FoxM1 trans-activation [62][4].
2. Strychnine/Brucine
Brucine is an alkaloid related to strychnine obtained from the Strychnic Nux-vomica tree. It has analgesic, anti-cancer, anti-inflammatory, antioxidant, and anti-venom properties [63][5]. In vitro, it inhibits the proliferation of Hela and K562 cell lines. Brucine also showed anti-metastasis action in MDA-MB-231 and Hs578-T-cells and inhibited invasive capacity and adhesion of MDA-MB-231 and Hs578-T-cells Matrigel, and preventing mRNA of E-cadherin, catenin, VIM, FN1, MMP-2, and MMP-9 in MDA-MB-231 cells [64][6]. These data collectively suggest that brucine might be a potential anti-cancer molecule; in vivo studies are still needed to confirm its anti-cancer potential.
3. Eugenol
A polypropanoid group of compounds is found in seeds of many plants, such as cloves, cinnamon, nutmeg, and bay leaves. It has antioxidant, anti-bacterial, anti-inflammatory, and anti-cancer activity and is widely used as a cosmetic, perfume, and culinary ingredient. It has anti-cancer potential due to its ability to increase reactive oxygen species (ROS) formation and apoptotic action, increase Cyt C’s release, and inhibit the EMT process, limiting the cells’ ability to metastasize [65][7]. It has shown anti-cancer activity against malignancies, including leukemia, lung, colon, colorectal, skin, gastric, breast, cervical, and prostate cancer, through the processes described below in Table 31.
Table 31.
Mechanisms of Eugenol for anti-apoptotic in various cancers.
| Type of the Tumor | Study Type | Effective Dose | Mechanism | References |
|---|---|---|---|---|
| Lung cancer | In vitro | 1000 μM | Decrease cycloxygenase-2 activity, which leads to cell cycle arrest in the S phase followed by cell death | [66][8] |
| Colon cancer | In vitro | 800 μM | Boosts the cytotoxic effects of cisplatin and doxorubicin synergistically. | [67][9] |
| Gastric cancer | In vitro | Low conc. | Inhibits cancer growth by upregulating preinvasive and angiogenic molecules and favoring apoptosis via the mitochondrial pathway via altering Bcl-2 family proteins. | [68][10] |
| Cervical cancer | In vitro | 50–200 μM | Prevents the cell cycle and causes apoptosis, and inhibits DNA synthesis. | [69][11] |
| Breast cancer | In vitro | 2 μM | Suppresses breast cancer-related oncogenes by downregulating E2F1 and its downstream anti-apoptotic target | [70][12] |
4. Resveratrol
Resveratrol (RES) chemically trihydroxy stilbene is a polyphenol in grapes, berries, peanuts, and wine. RES has been shown to have cardioprotective, anti-inflammatory, and anti-aging effects. Studies also suggest that it also has anti-cancer properties. Moreover, RES has a regulatory role in EMT and the hedgehog (Hh) signaling pathway, which is critical for vertebrate development, homeostasis, and cancer. Hh is abnormally activated in breast, prostate, and pancreatic cancer (PC) and has a role in metastasis and invasion of GC via induction of the EMT. Hence, Hh signaling pathway is the center of attraction for anti-cancer activity. RES suppresses the Hh pathway, thus inhibiting cancer invasion and metastasis.
Moreover, RES has also been shown to block the Hh signaling pathway and EMT in malignancies [71][13]. A study proved that RES inhibited EMT in Glioblastoma (GBM) cells, as evidenced by morphological changes in the RES-treated G.B.M. cells. RES also inhibits EMT-mediated migration and invasion of GBM cells and EMT-induced stem cell-like properties in GBM cells [72][14]. A TGF-β/Smad signaling pathway is associated with the proliferation, differentiation, and migration of the cells and promotes results in invasion and metastasis. RES inhibited the penetration and metastasis by EMT-induced phosphorylation of Smad2 and Smad3 in a dose-dependent manner, suggesting the function of RES on EMT is related to Smad-dependent signaling [72][14].
5. Polyphyllin 1
Polyphyllin 1 (PP 1) demonstrated its anti-cancer activity via its apoptotic action and several pathways effectively against various cancers. Polyphyllin I induces apoptosis in HepF-2-Cells, and neural progenitor cells (NPC) cell lines [73][15]. The natural herb Paris polyphylla makes PP1 and has anti-cancer properties against various malignancies, including drug-resistant tumors. Paris polyphylla was recently found to inhibit CRC cells by activating autophagy and improving the efficiency of chemotherapy (Doxorubicin). By decreasing CIP2A/PP2A/Akt signaling, PP1 also reduced cisplatin-resistant GC cells. Liu et al. demonstrated that PP 1 has potent anti-cancer action on human non-small cell lung cancer (NSCLC) mediated by CHOP stabilization. PP1 induces ROS, and ER stress inhibits unfolded protein response (UPR) in cancer cells, subsequently increasing the levels of CHOP Via, accelerating CHOP gene expression. The UPR chaperone GRP78, restrained by PP1, is the main mechanism for CHOP stabilization [74][16].
6. Paeoniflorin (PF)
Paeoniflorin (PF) is a monoterpene glycoside derived from the root of Paeonia lactiflora. In the past, this plant’s roots were utilized in eastern medicine for pain, muscle spasms, inflammation, menstruation dysfunction, and degenerative illnesses for a long time [75,76,77,78][17][18][19][20]. Studies indicated that PF inhibits tumor growth, invasion, and metastasis in vivo and in vitro. In hypoxia-induced EMT in MDA-MB-231 BC cells, PF treatment resulted in a considerable increase in E-cadherin levels and a drop in CDH2 and Vimentin levels in the cells. Subsequently, it suppressed the EMT process by altering the expression of HIF-1, which is involved in hypoxia-driven EMT [79][21]. The hippo pathway plays a significant role in the progression of GC. This pathway is said to be dysregulated and thus contributes to gastric oncology and metastasis. Two important factors, yes associated protein (YAP1) and Transcriptional coactivator with PDZ-binding motif (TAZ), produce their metastatic effect via crosslinking with Notch, TGF-β, and Wnt/ β-catenin in GC. In GC, PF exerts its anti-cancer effect via regulation of the hippo signaling pathway and downregulating the effect of TAZ [80][22]. Further, there is a need to explore its potential in other cancers.
7. Halicondramine
Halicondramine (HCA) is a trisoxazole-containing macrolide from the marine sponge Chondrosia corticata [81][23]. It possesses antifungal and cytotoxic properties. It also has anti-proliferative activity against cancerous cells [82,83][24][25]. Modulation of the EMT is a key target for their action. Treatment with HCA significantly reduced the expression of MMP2 and 9, and CDH2. On the contrary, E-cadherin expression was significantly increased. HCA also inhibits the expression of PRL-3, a metastasis-associated marker, and PI3 kinase subunits p85 and p110 (PRL-3’s downstream targets). These findings imply that HCA inhibits EMT in human adenocarcinoma prostate cancer cells by modulating PRL-3 and downstream targets, such as PI3 kinase [48][26].
8. Ligustrazine
Ligustrazine (LSZ) is obtained from the rhizome of Ligusticum wallichii [84][27]. LSZ is shown to have anti-inflammatory, anti-fibrotic, antioxidant activity, and tumor-suppressing properties in numerous cancers, including LC, GC, BC, and melanoma [85][28]. LSZ showed anti-proliferative and anti-metastatic action [6][29]. LSZ increased E-cadherin expression while decreasing the mesenchymal indicators CDH2 and VIM expression. LSZ inhibits EMT in SK-OV-3 cells via modulating miR-211 expression [86][30].
9. Fucoidan
The Fucoidan (FC) is a polysaccharide obtained from brown seaweeds and has shown anti-proliferative action on BC cells, such as 4T1 and MDA-MB-231. It also lowered metastatic lung nodules in female Balb/c mice with 4T1 xenografts. The TGFRs molecular network is critical in controlling EMT in cancer cells. It was observed that FC efficiently reverses TGFR-induced EMT morphological alterations, increases epithelial markers, decreases mesenchymal markers and transcriptional repressor expression Twist, Snail, and Slug. Furthermore, fucoidan suppresses migration and invasion during EMT, implying that TGFR-mediated signaling is involved in BC cells [87][31].
10. Penisuloxazin A
Penisuloxazin A (PNSA) is a fungal mycotoxin that belongs to a new epipolythiodiketopiperazines (ETPs) possessing a rare 3H-spiro[benzofuran-2,2′-piperazine] ring system. PNSA prevented MDA-MB-231 cell adhesion to coated Matrigels containing several ECM components. Furthermore, after PNSA therapy, there is a transition from spindle-shaped or polygonal mesenchymal to flat polygonal epithelial-like cell morphology. These suggest that PNSA can prevent EMT in MDA-MB-231 cells [88][32]. PNSA is also considered a potent heat shock protein 90 (HSP90) inhibitor, a well-known N-terminal inhibitor binding to the ATP pocket of HSP90 in preventing BC cell metastasis. Multiple signaling pathways critical for cancer cell proliferation and metastasis can be disrupted by inhibiting HSP90 [89][33].
11. Sophocarpine
Sophocarpinr (SC) is one of the most active components of Sophora alopecuroides L, a tetracyclic quinolizidine alkaloid. SC has shown various pharmacological actions, including immunoregulatory, anti-inflammatory, and anti-nociceptive [90][34]. SC has also been shown to preserve heart function from ischemic reperfusion by inhibiting NF-kB and reducing hepatocyte steatosis via activation of the AMPK signaling pathway. Furthermore, in head and neck squamous cell carcinoma (HNSCC) cells, SC has shown anti-proliferative and anti-metastatic by inhibiting dicer-catalyzed miR-21 maturation and activation of the p38MAPK signaling pathway. SC also reduced the HNSCC tumor’s growth in vivo by reversing the EMT in cancer cells. In UM-SCC-22B and UM-SCC-47 cells, SC treatment reduced the expression of the Ki-67 and VIM while increasing E-cadherin’s expression [91][35]. These findings suggest that SC could be a promising lead drug for HNSCC.
12. Renieramycin M
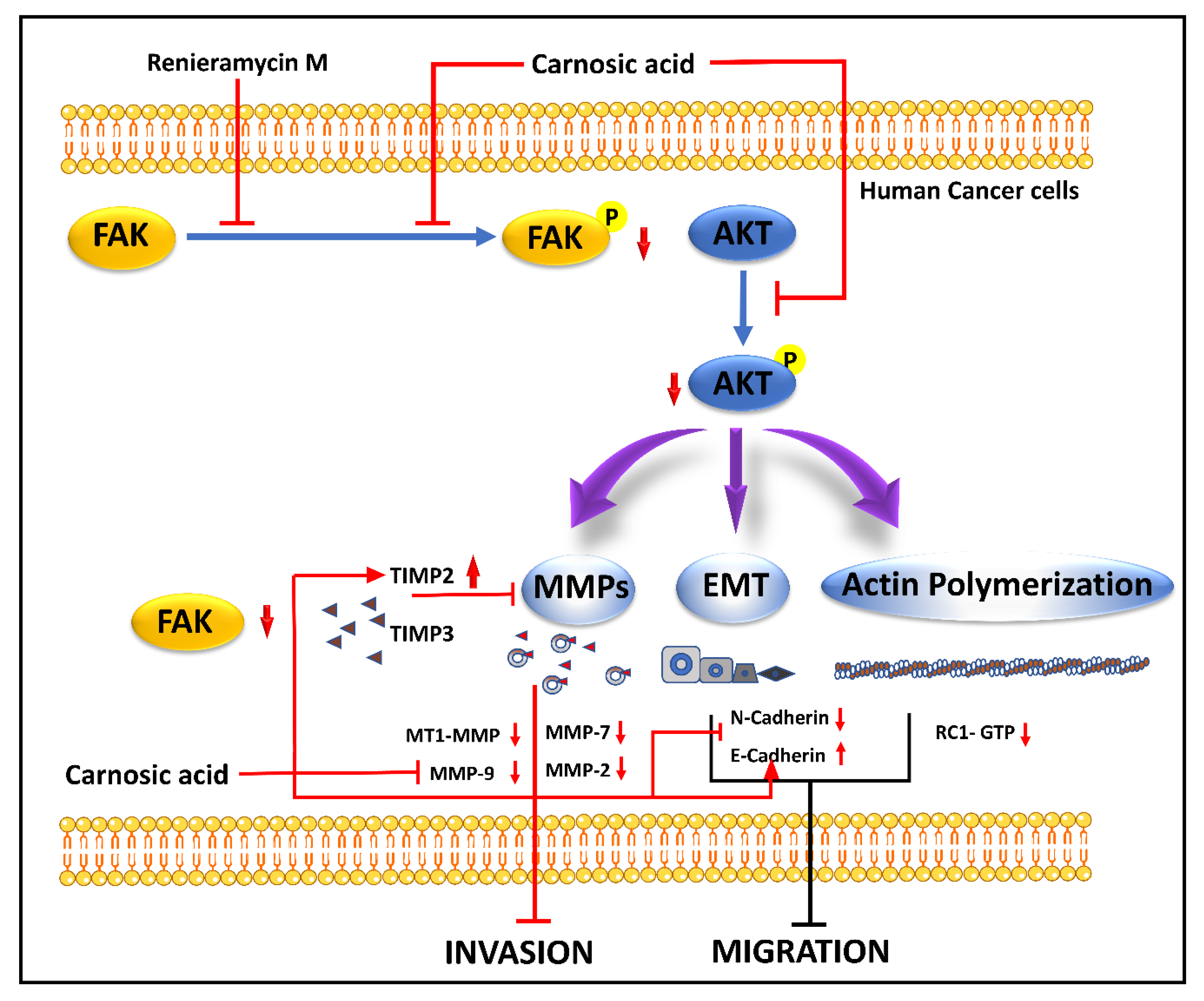
Renieramycin M (RM) (22-Boc-Gly-RM), produced by Xestospongia sp., is a semi-synthetic amino ester derivative of the bistetrahydroisoquinoline alkaloid. Studies suggested RM-mediated inhibition of anchorage-independent development and sensitization of detachment-induced cell death in human lung cancer cells [92][36]. A semi-synthetic derivative of RM with a hydroquinone amino ester extension was synthesized to retain cytotoxicity with increase cancer selectivity [93][37]. It hinders the phosphorylation of FAK and Akt molecules, which upregulate TIMP2 and TIMP3 and downregulate MMPs expression. The inhibition of the p-FAK/p-Akt signal also marks the downregulation of CDH2 and Rac1-GTP and the upregulation of E-cadherin, where the regulation of cytoskeleton regulatory protein (Rac1-GTP), MMP-associated molecules (TIMP2, TIMP3) [94][38]. The mechanism of action of RM is demonstrated in Figure 31.
13. Luteolin
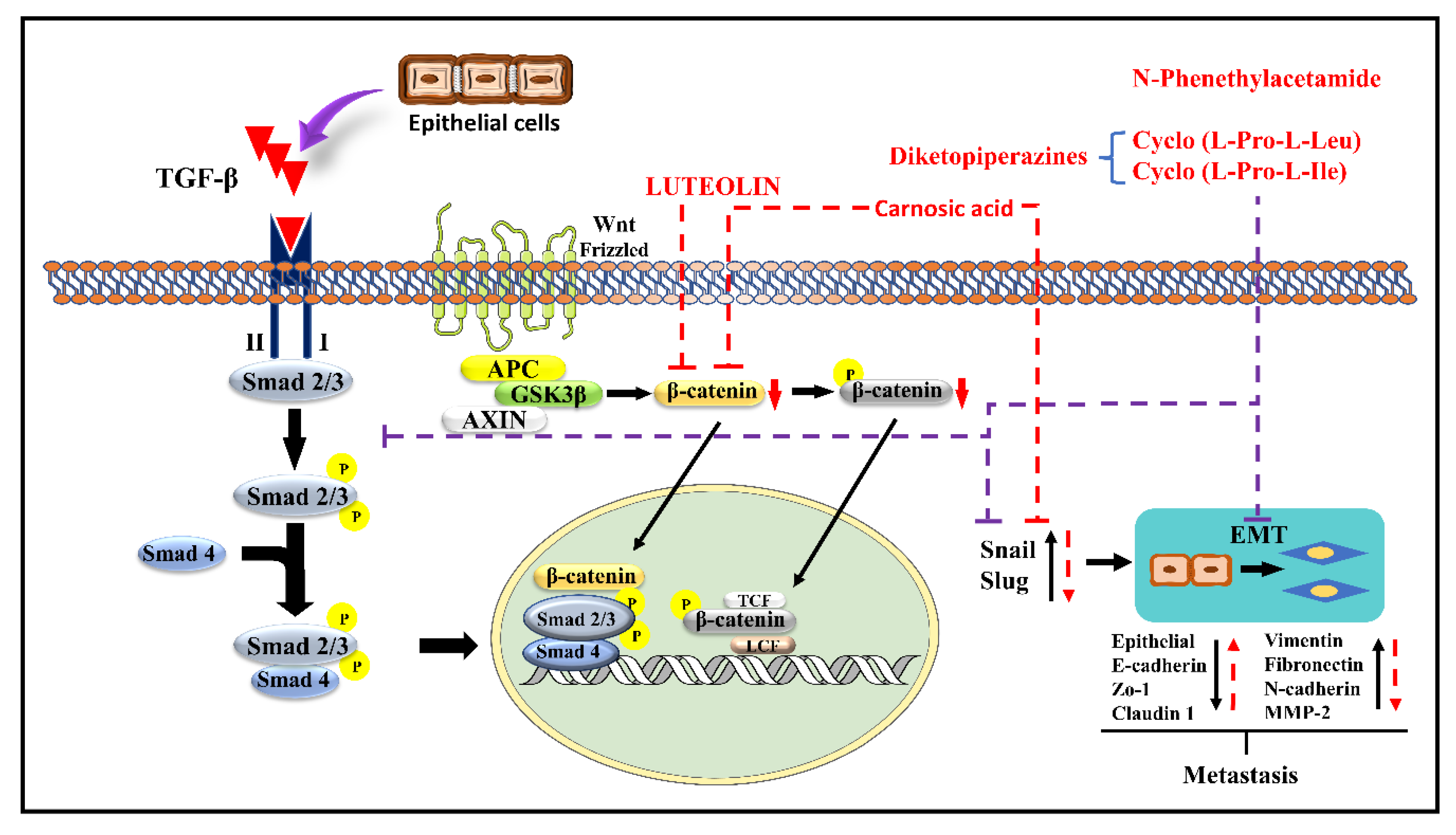
Luteolin (LT) (3,4,5,7-tetrahydroxy flavone) is a flavonoid in many plants including broccoli, carrots, perilla leaves, seeds, and celery. It possesses anti-allergy, anti-inflammatory, anti-cancer, antioxidant, and anti-microbial properties [96,97,98][40][41][42]. In various cancers (including lung, GBM, BC, CRC, PC), LT inhibits cell proliferation and tumor growth, promotes cancer cell apoptosis and cell cycle arrest, reduces drug resistance, and reduces cancer cell invasiveness and metastasis [99][43]. LT can also stop EMT from occurring, shrinking in the cytoskeleton, increasing the expression of E-cadherin, and decreasing the expression of CDH2, Snail, and VIM [100][44]. LT inhibits the Smad 2/3 pathway and the Wnt/-catenin pathway by inhibiting the synthesis of Snail and Slug by downregulating the production of β-catenin. Doing so prevents metastasis by upregulating CDH2, Zo 1, and claudin 1 and downregulating CDH2, fibronectin, VIM, and MMP-2 [99,101][43][45]. The mechanism of the action of LT is demonstrated in Figure 42.
14. Carnosic Acid
Carnosic acid (CA), a polyphenolic diterpene found in rosemary (Rosmarinus officinalis), has anti-cancer, anti-viral, and anti-inflammatory activities. CA suppresses cancer cell migration and proliferation while lowering vascular endothelial growth factor expression. In leukemia and CRC cells, CA also causes cell cycle arrest at the G2/M phase by downregulating cyclin expression and has been shown to trigger apoptotic cell death in human NB and PrC cells [102][46]. CA inhibits EMT and cell migration in B16F10 cells in a dose-dependent manner. It prevents Src/AKT phosphorylation and, therefore, activation. It decreases the secretion of uPAc, MMP-9, and TIMP-1, whereas it increases the secretion of TIMP-2 and has no effect on the secretion of MMP-2 and plasminogen activator inhibitor-1 (PAI-1). It is also responsible for the decrease in the expression of Snail and Slug but does not affect the expression of Twist in B16F10 melanoma cells [95][39]. The mechanism of action of CA is demonstrated in Figure 31 and Figure 42.
15. N-Phenethylacetamide
N-Phenethylacetamide (NPA) is found in the Aquamarina Sp. (MC085). Three compounds, two diketopiperazines [cyclo(L-Pro-L-Leu) (1) and cyclo(L-Pro-L-Ile) (2), and one NPA (3)] isolated with anti-cancer activity. By altering TGF-induced E.M.T., NPA inhibits the TGF/Smad pathway and suppresses A549 cell metastasis. It prevents Snail and Slug expression by inhibiting Smad 2/3 phosphorylation. It also suppresses Snail and Slug, which upregulates the epithelial markers E-cadherin, Zo-1, and claudin-1 while downregulating VIM, FN1, CDH2, and MMP-2 expression, preventing metastasis [101][45]. The mechanism of NPA is shown in Figure 42.
16. α-Solanine
α-Solanin (AS), a steroidal glycoalkaloid obtained from nightshade (Solanum nigrum Linn.), suppresses tumor cell growth and causes apoptosis in colon, liver, cervical, lymphoma, and stomach cancer cells. However, the mechanism by which it blocks cancer cell metastasis remains unknown. An animal model of BC induces cell death and inhibits cell proliferation and angiogenesis, resulting in chemotherapeutic actions [103,104][47][48]. It also increases E-cadherin expression, reducing VIM expression and cell invasion, which inhibits EMT. It also decreases extracellular inducer of matrix metalloproteinase (EMMPRIN), MMP-2, and MMP-9, increasing Cysteine-rich protein with Kazal motifs (RECK), TIMP-1, and TIMP-2 mRNA expression levels. It downregulated the phosphorylation of Akt, ERK1/2, and PI3K. Furthermore, it increases tumor suppressor miR-138 expression while decreasing oncogenic miR-21 expression [6,105][29][49].
17. Baicalein, Wogonin (WG), and Oroxylin-A (ORA)
Baicalein (BAI), wogonin (WG), and oroxylin-A (ORA) are present in a plant, namely Scutellaria baicalensis [105][49]. It has been reported that the extract of Scutellaria baicalensis has anti-tumor activity. A study reported that using the combination of BAI (65.8%), WG (21.2%), and ORA (13.0%) compounds against A549 lung adenoma cancer cells inhibited the EMT process significantly [105][49]. The Total Flavonoid Aglycones Extract (TFAE) isolated from Scutellaria baicalensis has shown inhibition against tumors by inducing apoptosis, mainly BAI, WG, and ORA [106][50]. It was reported that the TFAE of Scutellaria baicalensis has inhibited the EMT of A549 cells via PI3K/AKT-TWIST1 axis [105][49].
4.18. Coptidis Rhizoma
18. Coptidis Rhizoma
It was reported that the extract of Coptidis Rhizoma (CR) could inhibit the EMT process via the TGF-β signaling pathway [107][51]. It has been shown that 30% ethanol extract of Coptidis Rhizoma can inhibit cell migration and invasion via blocking E-cadherin and decreasing expression of vimentin, Snail, and ZEB2 [107][51]. It has a potential anti-metastatic effect and can be a candidate against cancer. The different pathways and proteins targeted by all natural products are summarized in Figure 53.
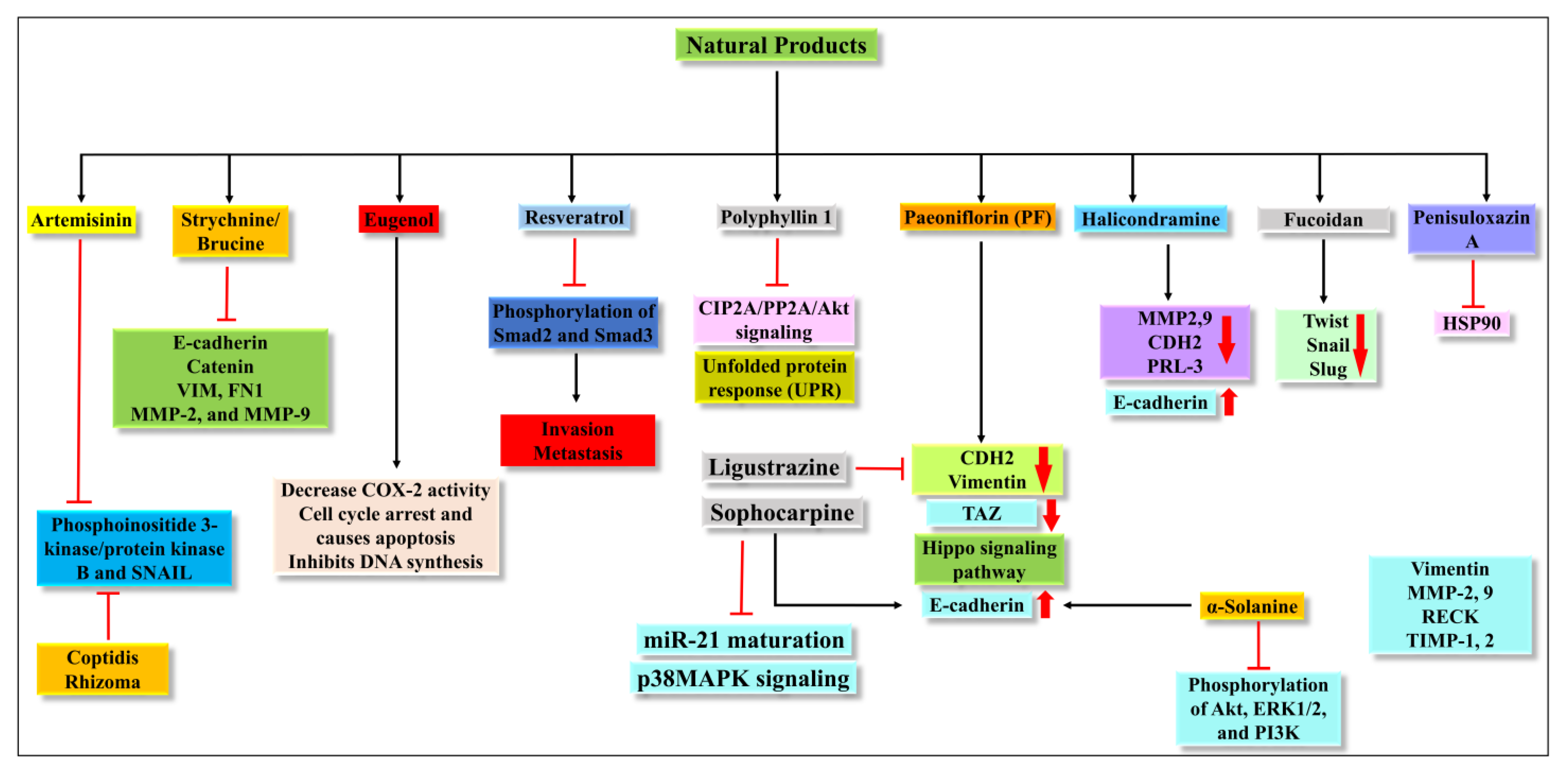
Figure 53.
Illustration of the different pathways targeted by the natural products.
References
- Anibogwu, R.; De Jesus, K.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995.
- Zhao, X.; Zhong, H.; Wang, R.; Liu, D.; Waxman, S.; Zhao, L.; Jing, Y. Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget 2015, 6, 5582–5596.
- Sun, Y.; Lu, X.; Li, H.; Li, X. Dihydroartemisinin inhibits IL-6-induced epithelial–mesenchymal transition in laryngeal squamous cell carcinoma via the miR-130b-3p/STAT3/β-catenin signaling pathway. J. Int. Med Res. 2021, 49, 030006052110094.
- Nandi, D.; Cheema, P.S.; Singal, A.; Bharti, H.; Nag, A. Artemisinin Mediates Its Tumor-Suppressive Activity in Hepatocellular Carcinoma Through Targeted Inhibition of FoxM1. Front. Oncol. 2021, 11, 751271.
- Lu, L.; Huang, R.; Wu, Y.; Jin, J.-M.; Chen, H.-Z.; Zhang, L.-J.; Luan, X. Brucine: A Review of Phytochemistry, Pharmacology, and Toxicology. Front. Pharmacol. 2020, 11, 377.
- Li, M.; Li, P.; Zhang, M.; Ma, F. Brucine suppresses breast cancer metastasis via inhibiting epithelial mesenchymal transition and matrix metalloproteinases expressions. Chin. J. Integr. Med. 2018, 24, 40–46.
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Eur. J. Mol. Clin. Med. 2020, 7, 3979.
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29.
- Petrocelli, G.; Farabegoli, F.; Valerii, M.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885.
- Manikandan, P.; Murugan, R.S.S.; Priyadarsini, R.V.; Vinothini, G.; Nagini, S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010, 86, 936–941.
- Kurnia, P.H.; Bachtiar, E.A.; Ria, Q.F.; Fadhil, F.; Anita, D. Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J. Appl. Pharm. Sci. 2021, 11, 49–53.
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 600.
- Gao, Q.; Yuan, Y.; Gan, H.-Z.; Peng, Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol. Lett. 2015, 9, 2381–2387.
- Song, Y.; Chen, Y.; Li, Y.; Lyu, X.; Cui, J.; Cheng, Y.; Zheng, T.; Zhao, L.; Zhao, G. Resveratrol Suppresses Epithelial-Mesenchymal Transition in GBM by Regulating Smad-Dependent Signaling. BioMed Res. Int. 2019, 2019, 1321973.
- Ahmad, B.; Rehman, S.U.; Azizullah, A.; Khan, M.F.; Din, S.R.U.; Ahmad, M.; Ali, A.; Tahir, N.; Azam, N.; Gamallat, Y.; et al. Molecular mechanisms of anticancer activities of polyphyllin VII. Chem. Biol. Drug Des. 2021, 97, 914–929.
- Liu, M.-M.; Zhu, M.-L.; Dong, R.-F.; Zhang, C.; Yang, L.; Kong, L.-Y.; Xia, Y.-Z. Polyphyllin I promotes cell death via suppressing UPR-mediated CHOP ubiquitination and degradation in non-small cell lung cancer. Chin. J. Nat. Med. 2021, 19, 255–266.
- Chen, Y.; Wu, K.; Wood, W.G. Paeonia lactiflora Extract Attenuating Cerebral Ischemia and Arterial Intimal Hyperplasia Is Mediated by Paeoniflorin via Modulation of VSMC Migration and Ras MEK/ERK Signaling Pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 482428.
- Wang, X.; Feng, S.; Wang, Y.; Chen, N.; Wang, Z. Phytomedicine Paeoniflorin: A neuroprotective monoterpenoid glycoside with promising anti-depressive properties. Phytomedicine 2021, 90, 153669.
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2019, 207, 107452.
- Ji, Y.; Dou, Y.-N.; Zhao, Q.-W.; Zhang, J.; Yang, Y.; Wang, T.; Xia, Y.-F.; Dai, Y.; Wei, Z.-F. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol. Sin. 2016, 37, 794–804.
- Zhou, Z.; Wang, S.; Song, C.; Hu, Z. Paeoniflorin prevents hypoxia-induced epithelial–mesenchymal transition in human breast cancer cells. OncoTargets Ther. 2016, 9, 2511–2518.
- Niu, K.; Liu, Y.; Zhou, Z.; Wu, X.; Wang, H.; Yan, J. Antitumor Effects of Paeoniflorin on Hippo Signaling Pathway in Gastric Cancer Cells. J. Oncol. 2021, 2021, 4724938.
- Chill, L.; Yosief, T.; Kashman, Y. Halichondramine, a New Tetracyclic Bipiperidine Alkaloid from the Marine Sponge Halichondria sp. J. Nat. Prod. 2002, 65, 1738–1741.
- Halim, H.; Chunhacha, P.; Suwanborirux, K.; Chanvorachote, P. Anticancer and antimetastatic activities of renieramyein M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anti-Cancer Res. 2011, 31, 193–201.
- Bae, S.Y.; Kim, G.D.; Jeon, J.-E.; Shin, J.; Lee, S.K. Anti-proliferative effect of (19Z)-halichondramide, a novel marine macrolide isolated from the sponge Chondrosia corticata, is associated with G2/M cell cycle arrest and suppression of mTOR signaling in human lung cancer cells. Toxicol. Vitr. 2013, 27, 694–699.
- Shin, Y.; Kim, G.D.; Jeon, J.-E.; Shin, J.; Lee, S.K. Antimetastatic Effect of Halichondramide, a Trisoxazole Macrolide from the Marine Sponge Chondrosia corticata, on Human Prostate Cancer Cells via Modulation of Epithelial-to-Mesenchymal Transition. Mar. Drugs 2013, 11, 2472–2485.
- Chen, J.; Wang, W.; Wang, H.; Liu, X.; Guo, X. Combination treatment of ligustrazine piperazine derivate DLJ14 and adriamycin inhibits progression of resistant breast cancer through inhibition of the EGFR/PI3K/Akt survival pathway and induction of apoptosis. Drug Discov. Ther. 2014, 8, 33–41.
- Xie, H.-J.; Zhao, J.; Zhuo-Ma, D.; Zhan-Dui, N.; Er-Bu, A.; Tsering, T. Inhibiting tumour metastasis by DQA modified paclitaxel plus ligustrazine micelles in treatment of non-small-cell lung cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3465–3477.
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González-Mateo, G.T. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715.
- Zhang, H.; Ding, S.; Xia, L. Ligustrazine inhibits the proliferation and migration of ovarian cancer cells via regulating miR-211. Biosci. Rep. 2021, 41, BSR20200199.
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGF receptor degradation in breast cancer. Carcinogenesis 2012, 34, 874–884.
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118.
- Zhang, A.; Qi, X.; Du, F.; Zhang, G.; Li, D.; Li, J. PNSA, A Novel C-Terminal Inhibitor of HSP90, Reverses Epithelial–Mesenchymal Transition and Suppresses Metastasis of Breast Cancer Cells In Vitro. Mar. Drugs 2021, 19, 117.
- Gao, Y.; Li, G.; Li, C.; Zhu, X.; Li, M.; Fu, C.; Li, B. Anti-nociceptive and anti-inflammatory activity of sophocarpine. J. Ethnopharmacol. 2009, 125, 324–329.
- Liu, W.; Zhang, B.; Chen, G.; Wu, W.; Zhou, L.; Shi, Y.; Zeng, Q.; Li, Y.; Sun, Y.; Deng, X.; et al. Targeting miR-21 with Sophocarpine Inhibits Tumor Progression and Reverses Epithelial-Mesenchymal Transition in Head and Neck Cancer. Mol. Ther. 2017, 25, 2129–2139.
- Prateep, A.; Sumkhemthong, S.; Karnsomwan, W.; De-Eknamkul, W.; Chamni, S.; Chanvorachote, P.; Chaotham, C. Avicequinone B sensitizes anoikis in human lung cancer cells. J. Biomed. Sci. 2018, 25, 32.
- Chamni, S.; Sirimangkalakitti, N.; Chanvorachote, P.; Suwanborirux, K.; Saito, N. Chemistry of renieramycins. Part 19: Semi-syntheses of 22-O-amino ester and hydroquinone 5-O-amino ester derivatives of renieramycin M and their cytotoxicity against non-small-cell lung cancer cell lines. Mar. Drugs 2020, 18, 418.
- Oo, Y.; Nealiga, J.Q.L.; Suwanborirux, K.; Chamni, S.; Ecoy, G.A.U.; Pongrakhananon, V.; Chanvorachote, P.; Chaotham, C. 22-O-(N-Boc-l-glycine) ester of renieramycin M inhibits migratory activity and suppresses epithelial–mesenchymal transition in human lung cancer cells. J. Nat. Med. 2021, 75, 949–966.
- Park, S.Y.; Song, H.; Sung, M.-K.; Kang, Y.-H.; Lee, K.W.; Park, J.H.Y. Carnosic Acid Inhibits the Epithelial-Mesenchymal Transition in B16F10 Melanoma Cells: A Possible Mechanism for the Inhibition of Cell Migration. Int. J. Mol. Sci. 2014, 15, 12698–12713.
- Chung, J.G.; Wu, L.T.; Chang, S.H.; Lo, H.H.; Hsieh, S.E.; Li, Y.C. Inhibitory Actions of Berberine on Growth and Arylamine N-Acetyltransferase Activity in Strains of Helicobacter Pylori from Peptic Ulcer Patients. Int. J. Toxicol. 1999, 18, 35–40.
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646.
- Chen, C.-Y.; Peng, W.-H.; Tsai, K.-D.; Hsu, S.-L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614.
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2016, 37, 895–902.
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.A.; Khan, I.A.; Imran, A.; Orhan, I.E.E.; Rizwan, M.; Atif, M.; et al. luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612.
- Lee, M.J.; Kim, G.J.; Shin, M.-S.; Moon, J.; Kim, S.; Nam, J.-W.; Kang, K.S.; Choi, H. Chemical Investigation of Diketopiperazines and N-Phenethylacetamide Isolated from Aquimarina sp. MC085 and Their Effect on TGF-β-Induced Epithelial–Mesenchymal Transition. Appl. Sci. 2021, 11, 8866.
- D’Alesio, C.; Bellese, G.; Gagliani, M.C.; Aiello, C.; Grasselli, E.; Marcocci, G.; Bisio, A.; Tavella, S.; Daniele, T.; Cortese, K.; et al. Cooperative antitumor activities of carnosic acid and Trastuzumab in ERBB2+ breast cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 154.
- Lin, K.; Lin, C.; Kuo, S.; Lai, J.; Wang, Y.; You, H.; Hsu, M.; Chen, C.; Shiu, L. Carnosic acid impedes cell growth and enhances anti-cancer effects of carmustine and lomustine in melanoma. Biosci. Rep. 2018, 38, BSR20180005.
- Shen, K.-H.; Liao, A.C.-H.; Hung, J.-H.; Lee, W.-J.; Hu, K.-C.; Lin, P.-T.; Liao, R.-F.; Chen, P.-S. α-Solanine Inhibits Invasion of Human Prostate Cancer Cell by Suppressing Epithelial-Mesenchymal Transition and MMPs Expression. Molecules 2014, 19, 11896–11914.
- Cao, H.-J.; Zhou, W.; Xian, X.-L.; Sun, S.-J.; Ding, P.-J.; Tian, C.-Y.; Tian, F.-L.; Jiang, C.-H.; Fu, T.-T.; Zhao, S.; et al. A Mixture of Baicalein, Wogonin, and Oroxylin-A Inhibits EMT in the A549 Cell Line via the PI3K/AKT-TWIST1-Glycolysis Pathway. Front. Pharmacol. 2022, 12, 4171.
- Wang, Y.; Cao, H.-J.; Sun, S.-J.; Dai, J.-Y.; Fang, J.-W.; Li, Q.-H.; Yan, C.; Mao, W.-W.; Zhang, Y.-Y. Total flavonoid aglycones extract in Radix scutellariae inhibits lung carcinoma and lung metastasis by affecting cell cycle and DNA synthesis. J. Ethnopharmacol. 2016, 194, 269–279.
- Kang, Y.-H.; Wang, J.-H.; Lee, J.-S.; Lee, N.-H.; Son, C.-G. Coptidis Rhizoma Suppresses Metastatic Behavior by Inhibiting TGF-β-Mediated Epithelial-Mesenchymal Transition in 5-FU-Resistant HCT116 Cells. Front. Pharmacol. 2022, 13, 2336.
More
