Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Francesco Nappi.
Bicuspid aortic valve (BAV) is the most frequent congenital cardiac disease. Alteration of ascending aorta diameter is a consequence of shear stress alterations due to haemodynamic abnormalities developed from inadequate valve cusp coaptation.
- bicuspid aortic valve
- aortopathy
- classification
1. Introduction
Bicuspid aortic valve (BAV) is the most frequent congenital cardiac pathology; has a prevalence of 1–2% [1], a high incidence of adverse outcomes, especially aortic stenosis (AS) and aortic regurgitation (MR) [2]; and is at least three times more common in males than females [3].
Bicuspid aortopathy, reported in 50% of BAV patients, consists of the aorta enlargement starting from the aortic root and involving the aortic arch and depends on blood flux turbulences characterized by power vectors directed against the aortic toot and the convexity of the vessel [4,5,6,7][4][5][6][7]. Recently, micro-RNA (miRNA) has been studied regarding post-transcriptional regulation of genes in aortopathy manifestation. [8,9][8][9].
2. Genetics and Molecular Biology
Estimating mutation genes and their inheritance patterns is challenging [7] because locus 9q34.3 alteration causes mutations in regulators NOTCH1 with secondary pathological aortic valve development [10,11][10][11]; gene damages on 18q, 5q, and 13q induces BAV [12]; and finally, damages to the smooth muscle alfa actine (ACTA 2) gene produce BAV and aortic aneurysms [13]. There is a tight linkage between BAV expression and other congenital pathologies such as the coarctation of the aorta. Concerning BAV phenotype, Shone’s syndrome with a left-sided lesion that can cause inflow and outflow obstruction, Turner’s syndrome with aortic coarctation, and William’s syndrome involving supravalvular stenosis may be observed. Moreover, ventricular septal defect, atrial septal defect, patent ductus arteriosus, and coronary vessels, which may mainly involve single coronary and reversal coronary dominance, have been reported [14,15,16][14][15][16]. Micro-RNAs (MiRNAs) need to be considered in biochemical and molecular changes in BAV and aorthopathy (Table 21). MiRNAs are small, single-stranded, noncoding RNA molecules that determine the post-transcriptional regulation of gene expression. The effects of miRNAs are the result of base pairing with complementary sequences within mRNA molecules that are silenced by cleavage of the mRNA strand, destabilization of the mRNA by shortening its tail, and less efficient translation into proteins by ribosomes [17]. MiRNA expression profiling studies show that the expression levels of certain miRNAs change in diseased human hearts, suggesting their involvement in cardiomyopathies. MiR-712 is a potential predictor of atherosclerosis, has blood flow-dependent expression, and miR-712 is also upregulated in endothelial cells exposed to naturally occurring d-flow in the greater curvature of the aortic arch [18]. Several studies have investigated the cooperation of miRNA, metalloproteinases (MMP), and tissue inhibitor of matrix metalloproteinases (TIMP) in aorthopathy secondary to morphological alteration of the aortic valve. miRNAs related to dilation of the thoracic aorta (TA) are upregulated in transcriptional and epigenetic ways: different levels of MMP-2, MMP-9, TIMP-1, and TIMP-9 were observed [19]. A high level of MMP-2 and increased levels of miR-17 and miRNAs with the same genetic features as miR-17 were found in a comparative study involving patients with mild and severe aorta dilation, with a decreased level of TIMP -1, TIMP-2, and TIMP-3, thus hypothesizing a continuous development of TA influenced by BAV [20]. A recent study showed a relationship between miR-133a and TIMP-1 and TIMP-2 without reporting a statistically significant association between miR-143 and MMP-2 [21].Table 21. Gene expression involved in valve and aortic diseases. ACTA 2: alfa actine 2, AXIN: gene encodes a cytoplasmic protein that contains a regulation of G-protein signaling (RGS) domain and a disheveled and axin (DIX) domain, BAV: bicuspid aortic valve, ENG: Endoglin, FBN1: fibrillin 1, GATA (sequence for transcription factors for zinc proteins’ binding DNA sequence), NOS3: nitric oxide synthase 3, NOTCH1 (gene encoding transmembrane proteins), PDIA2: protein disulfide isomerase family A member 2, PECAM-1: platelet endothelial cell adhesion molecule-1, TGF: transforming growth factor, TIMP: tissue inhibitor of matrix metalloproteinases.
| Gene Expression | Pathology |
|---|---|
| miR-146-5p | BAV, aortic aneuurysm (convex region) |
| miR-21-5p | BAV, aortic aneuurysm (convex region) |
| miR-17 | Aoritc anurysm |
| miR 21 | Aortic aneurysm |
| miR-34 a | Aortic aneurysm |
| miR-122 | BAV |
| miR 130 a | BAV |
| miR-133a | TIMP1,TIMP2, aortic aneurysm |
| mi-R 143 | Aortic aneurysm |
| mi-R 145 | Aortic aneurysm |
| miR 146-5p | Aortic aneurysm |
| miR-200 | Endothelial-mesenchimal/epithelial mesenchimal |
| miR-423-5p | BAV, aortic aneurysm |
| miR-424-3p downregulation | Cell proliferation, apoptosis, endothelial cells alterations, aortic anuerysm |
| miR-486 | BAV |
| miR-494 | PECAM |
| miR-712 | Atherosclerosis, aortic aneurysm |
| miR-718 | Aortic aneurysm |
| ACTA2 | BAV. Aortic aneurysm |
| AXIN1-PDIA2 | BAV |
| ENG | BAV |
| FBN 1 | BAV |
| GATA4/GATA5/GATA6 | BAV |
| NOS3 | BAV |
| NOTCH1 (9q34.3) | BAV, outflow tract malformation |
| TGFb1/TGFb2 | Sporadic BAV, Loeys-Dietz syndrome |
| 18q | BAV |
| 5q | BAV |
| 13q | BAV |
3. Classification and Nomenclature
Since 1970, several classifications of BAV, derived from pathology, US scan, CT scan, and MR patterns (Table 32), have been proposed [36]. Recently, an international consensus statement developed a classification based on the progression of cusps fusion and geometry of commissurae [37], with particular attention to surgical indications and techniques.Table 32. BAV classifications (adapted from Michelena HI et al./European Journal of cardio-thoracic surgery). Abbreviations; BAV, bicuspid aortic valve; BAVCon, bicuspid aortic valve consortium; LN, left non-coronary fusion; RL, right–left fusion; RN, right non-coronary fusion.
| Author | Nomenclature |
|---|---|
| Roberts [36] 1970 | Anterior–posterior cusps Right–left cusps Presence of raphe |
| Brandenburg et al. [38] 1983 | Clock-face nomenclature: Commissures at 4–10 o’clock with raphe at 2 o’clock (R-L) Commissures at 1–6 o’clock with raphe at 10 o’clock (RN) Commissures at 3–9 o’clock without raphe (L-N) |
| Angelini et al. [39] 1989 | Anterior–posterior cusps Right–left cusps Presence of raphe |
| Sabet et al. [40] 1999 | RL RN LN Presence of raphe |
| Sievers and Schmidtke [41] 2007 | Type 0 (no raphe): anteroposterior or lateral cusps (true BAV) Type 1 (1 raphe): R-L, RN, L-N Type 2 (2 raphes): L-R, RN |
| Schaefer et al. [42] 2008 | Type 1: RL Type 2: RN Type 3: LN Presence of raphe Aorta: Type N: normal shape Type E: sinus effacement Type A: ascending aorta dilatation |
| Kang et al. [43] 2013 | Anteroposterior orientation: type 1: R-L with raphe type; 2: R-L without raphe Right–left orientation: Type 3: RN with raphe Type 4: L-N with raphe Type 5: symmetrical cusps with 1 coronary artery originating from each cusp Aorta: Type 0: normal Type 1: dilated root Type 2: dilated ascending aorta Type 3: diffuse involvement of the ascending aorta and arch |
| Michelena et al. [44] 2014 | BAVCon nomenclature: Type 1: R-L Type 2: RN Type 3: L-N Presence of raphe |
| Jilaihawi et al. [45] 2016 | Tricommissural: functional or acquired bicuspidity of a trileaflet valve Bicommissural with raphe Bicommissural without raphe |
| Sun et al. [46] 2017 | Dichotomous nomenclature: R-L Mixed: (RN or L-N) |
| Murphy et al. [47] 2017 | Clock-face nomenclature: Type 0: partial fusion/eccentric leaflet? Type 1: RN, RL, LN partial fusion/eccentric leaflet? Type 2: RL and RN, RL and LN, RN and LN partial fusion/eccentric leaflet? |
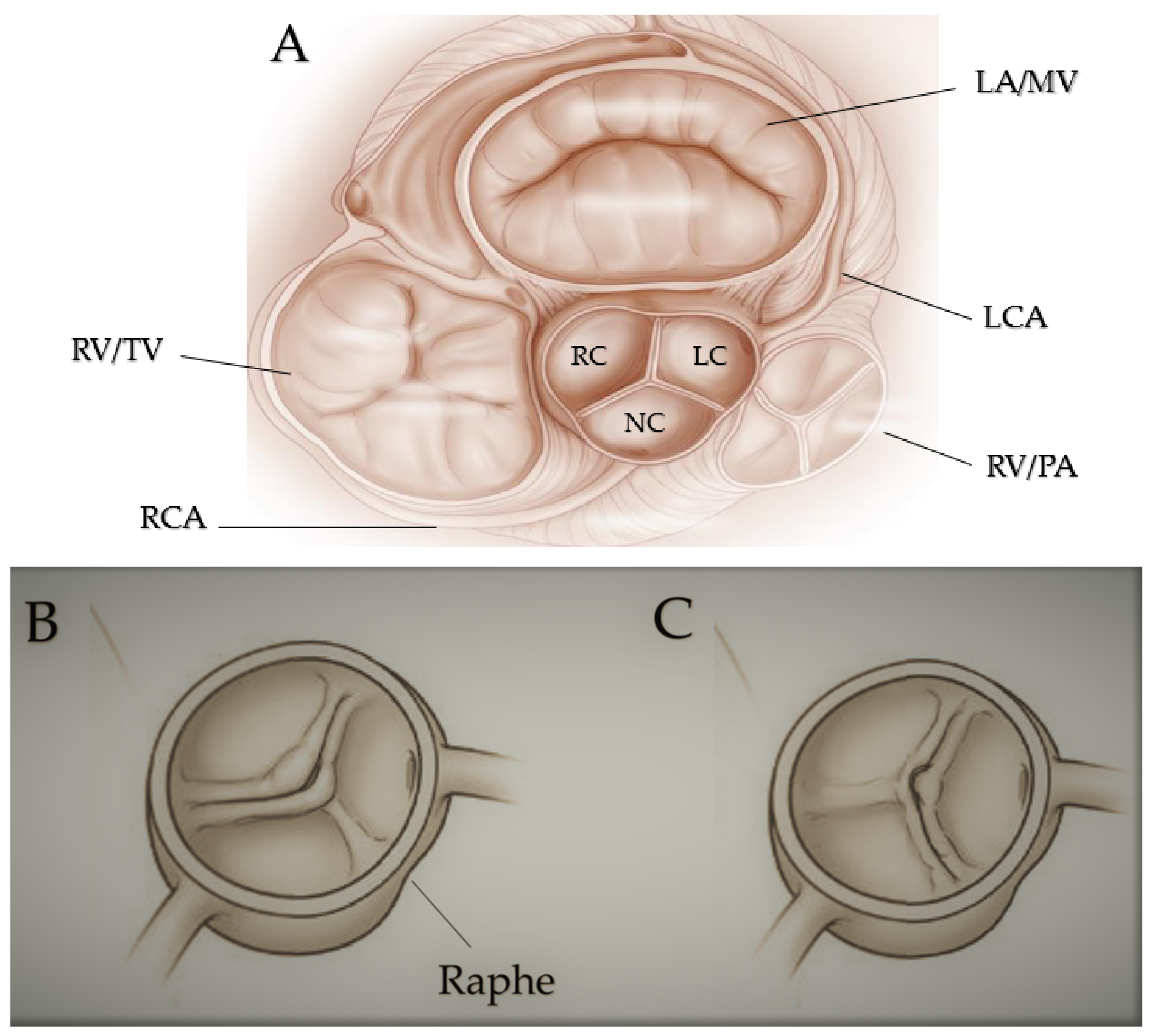
Figure 1. Fused bicuspid aortic valve. (A) Represents short-axis normal tricuspidal aortic pattern with anatomical proximities. Cusps’ fusion patterns seen in short heart axis: right-left coronary fusion (B), right-non coronary fusion (C). All BAVs have three sinuses. Raphe structure is between the fused cusps. Non-fused cusp is prominent in respect to the fused ones. The commissure angle of the non-fused cusp has a degree < 180°. Abbreviations: LA, left atrium; LC, left cusp; LCA, left coronary artery; MV, mitral valve; NC, non-coronary cusp; PA, pulmonary artery; RA, right atrium; RC, right cusp; RCA, right coronary artery; RV, right ventricule; TV, tricuspidalic valve. Licenses Centre Cardiologique du Nord; order date 8 September 2022; order number 5384080341542; publication NEJM; Title: Mitral valve Repair for Mitral valve prolapse.
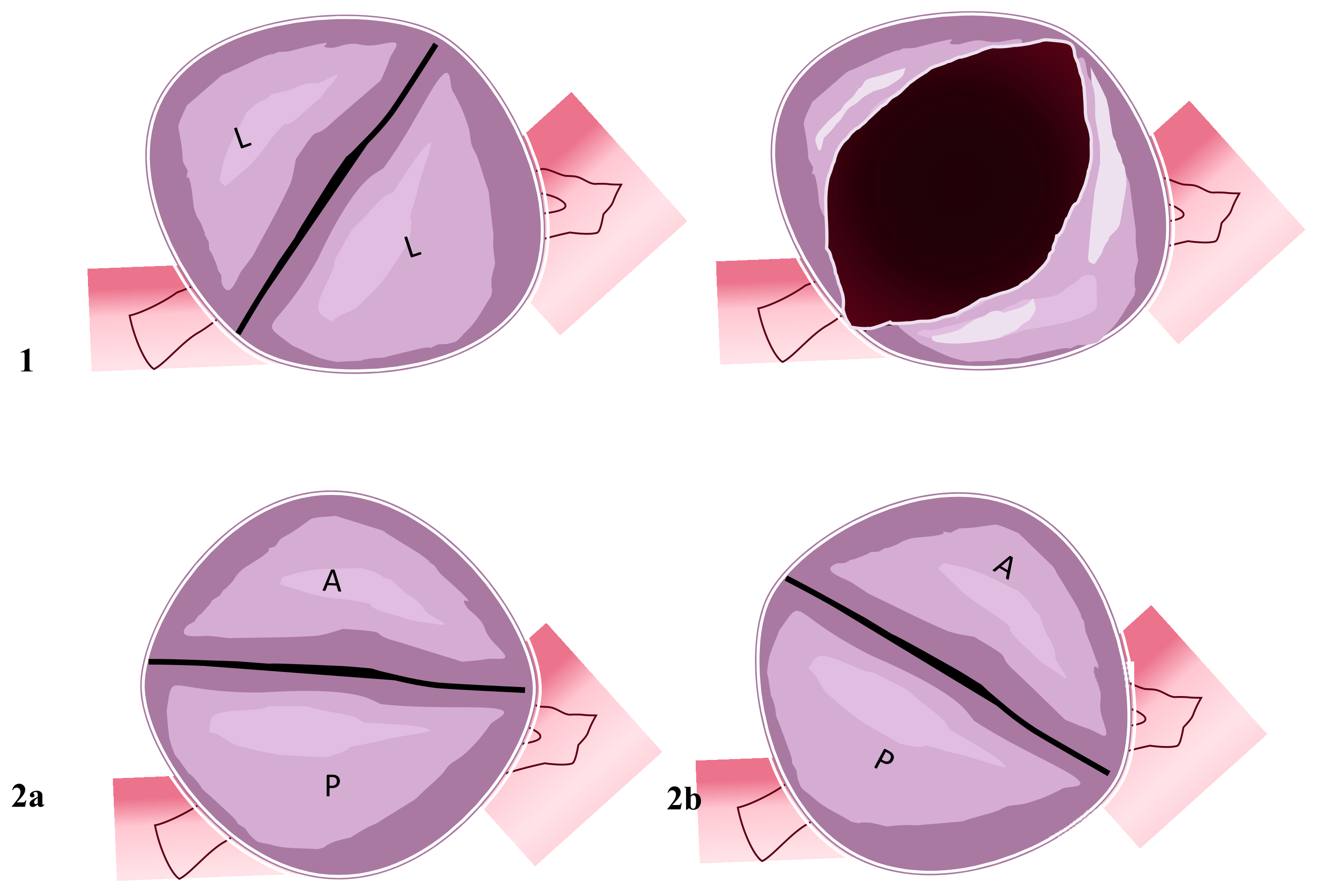
Figure 2. Two-sinus bicuspid aortic valve. Figure represents two cusps’ non-fusion patterns seen in the short heart axis. Aortic valves have two sinuses with two leaflets non-derived from fusion mechanisms. (1) Coronary arteries originate from the two sinuses with two lateral leaflets. The opened valve in systole phase has the oval-ball image. (2a) In this position, coronary arteries originate from the anterior sinus (right coronary artery) and posterior sinus (common left stem). (2b) Right coronary artery and common left stem both originate from anterior sinus. Abbreviations; A, anterior; L, lateral P, posterior.
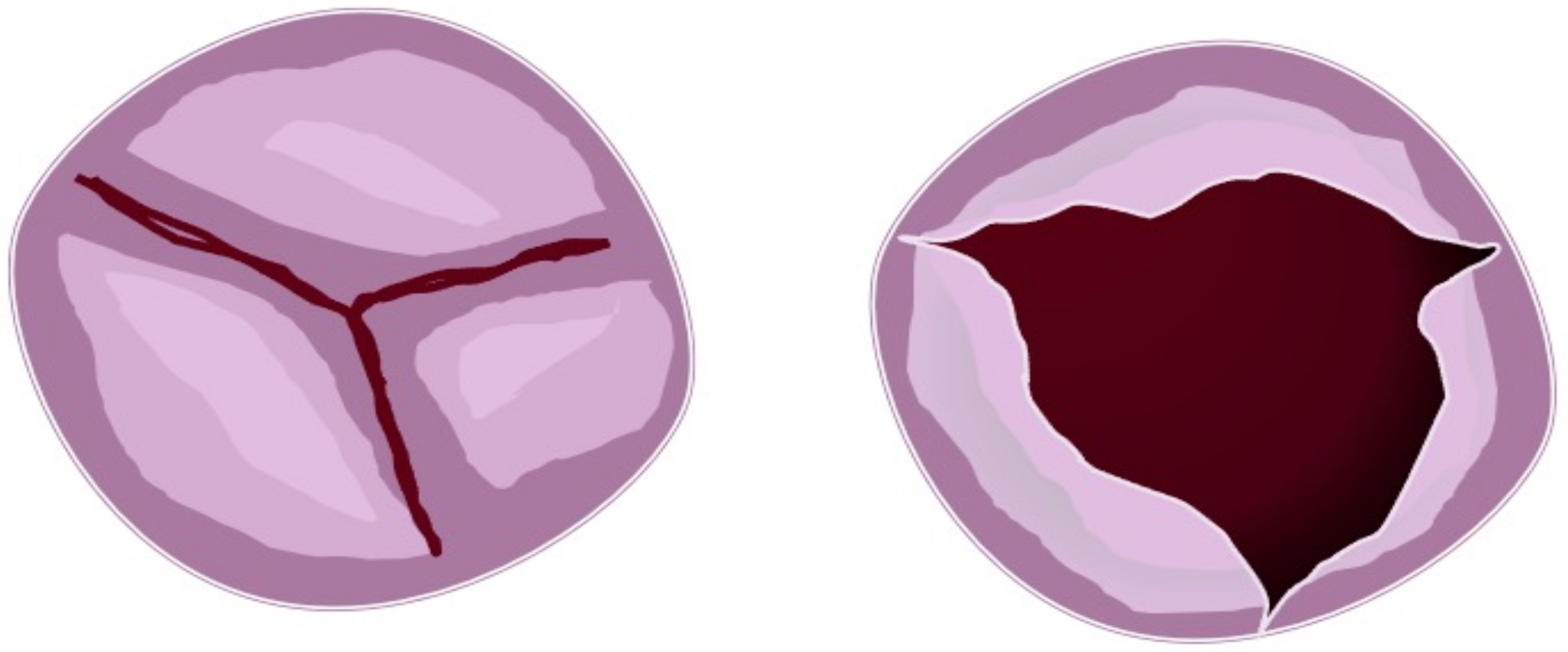
Figure 3. Partial fusion aortic valve. Figure represents three cusps with partial leaflets fusion seen in the short heart axis (left). In this case, the opened (right) aortic valve is similar to the normal valve but with a more narrow area.
4. BAV Geometry Types and Surgical Implications
In every subtype of the previous classification, we can identify the BAV geometrical pattern by evaluating the position of commissures related to the aortic orifice, their angle in the coaptation zone, the presence of raphe, and the morphology and the area of the cusps. In the fused BAV type, it is relevant to establish the relationships between the fused cusps and non-fused cusp and the angle of the commissures of the non-fused cusps (Figure 4).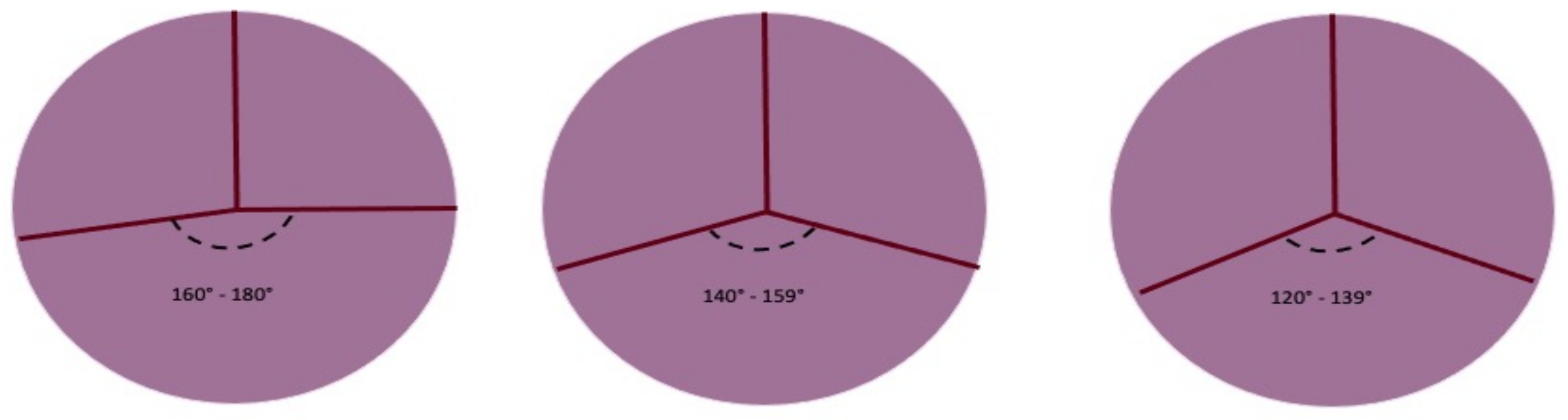
Figure 4. Symmetry of fused bicuspid aortic valve (adapted from Michelena HI et al./European Journal of cardio-thoracic surgery). Figure represents the angles determined by aortic valve leaflets fusion patterns. The length of raphe causes the retraction of fused leaflets and the non-physiological coaptation of the non-fused leaflet with fused leaflets. The geometry of the three patterns can be summarized as symmetrical, asymmetrical, and very asymmetrical The degree of the angle is important for surgical technique.
References
- Ward, C. Clinical significance of the bicuspid aortic valve. Heart 2000, 83, 81–85.
- Tzemos, N.; Therrien, J.; Yip, J.; Thanassoulis, G.; Tremblay, S.; Jamorski, M.T.; Webb, G.D.; Siu, S.C. Outcomes in adults with bicuspid aortic valves. JAMA 2008, 300, 1317–1325.
- Tutar, E.; Ekici, F.; Atalay, S.; Nacar, N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am. Heart J. 2005, 150, 513–515.
- Carro, A.; Teixido-Tura, G.; Evangelista, A. Aortic Dilatation in Bicuspid Aortic Valve Disease. Rev. Esp. Cardiol. 2012, 65, 977–981.
- Fedak, P.; Verma, S.; David, T.E.; Leask, R.; Weisel, R.D.; Butany, J. Clinical and Pathophysiological Implications of a Bicuspid Aortic Valve. Circulation 2002, 106, 900–904.
- Girdauskas, E.; Borger, M.; Secknus, M.-A.; Girdauskas, G.; Kuntze, T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur. J. Cardio-Thorac. Surg. 2011, 39, 809–814.
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800.
- Jia, H.; Kang, L.; Ma, Z.; Lu, S.; Huang, B.; Wang, C.; Zou, Y.; Sun, Y. MicroRNAs involve in bicuspid aortic aneurysm: Pathogenesis and biomarkers. J. Cardiothorac. Surg. 2021, 16, 230.
- Nappi, F.; Iervolino, A.; Singh, S.S.A.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132.
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274.
- Mohamed, S.A.; Aherrahrou, Z.; Liptau, H.; Erasmi, A.W.; Hagemann, C.; Wrobel, S.; Borzym, K.; Schunkert, H.; Sievers, H.H.; Erdmann, J. Novel missense mutations (p. T596M and p. P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem. Biophys. Res. Commun. 2006, 345, 1460–1465.
- Martin, L.; Ramachandran, V.; Cripe, L.H.; Hinton, R.B.; Andelfinger, G.; Tabangin, M.; Shooner, K.; Keddache, M.; Benson, D.W. Evidence in favor of linkage to human chromosomal regions 18q, 5q and 13q for bicuspid aortic valve and associated cardiovascular malformations. Hum. Genet. 2007, 121, 275–284.
- Guo, D.-C.; Pannu, H.; Tran-Fadulu, V.; Papke, C.L.; Yu, R.K.; Avidan, N.; Bourgeois, S.; Estrera, A.L.; Safi, H.J.; Sparks, E.; et al. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007, 39, 1488–1493.
- Higgins, C.B.; Wexler, L. Reversal of dominance of the coronary arterial system in isolated aortic stenosis and bicuspid aortic valve. Circulation 1975, 52, 292–296.
- Hutchins, G.M.; Nazarian, I.H.; Bulkley, B.H. Association of left dominant coronary arterial system with congenital bicuspid aortic valve. Am. J. Cardiol. 1978, 42, 57–59.
- Rashid, A.; Saucedo, J.F.; Hennebry, T.A. Association of Single Coronary Artery and Congenital Bicuspid Aortic Valve with Review of Literature. J. Interv. Cardiol. 2005, 18, 389–391.
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233.
- Insull, W., Jr. The Pathology of Atherosclerosis: Plaque Development and Plaque Responses to Medical Treatment. Am. J. Med. 2009, 122, S3–S14.
- LeMaire, S.A.; Wang, X.; Wilks, J.A.; Carter, S.A.; Wen, S.; Won, T.; Leonardelli, D.; Anand, G.; Conklin, L.D.; Wang, X.L.; et al. Matrix metalloproteinases in ascending aortic aneurysms: Bicuspid versus trileaflet aortic valves1. J. Surg. Res. 2005, 123, 40–48.
- Wu, J.; Song, H.-F.; Li, S.-H.; Guo, J.; Tsang, K.; Tumiati, L.; Butany, J.; Yau, T.M.; Ouzounian, M.; Fu, S.; et al. Progressive Aortic Dilation Is Regulated by miR-17–Associated miRNAs. J. Am. Coll. Cardiol. 2016, 67, 2965–2977.
- Naito, S.; Petersen, J.; Sequeira-Gross, T.; Neumann, N.; Escobar, J.D.; Zeller, T.; Reichenspurner, H.; Girdauskas, E. Bicuspid aortopathy—Molecular involvement of microRNAs and MMP-TIMP. Biomarkers 2020, 25, 711–718.
- Zhang, H.; Liu, D.; Zhu, S.; Wang, F.; Sun, X.; Yang, S.; Wang, C. Plasma Exosomal Mir-423-5p Is Involved in the Occurrence and Development of Bicuspid Aortopathy via TGF-beta/SMAD2 Pathway. Front. Physiol. 2021, 12, 759035.
- Zheng, R.; Zhu, P.; Gu, J.; Ni, B.; Sun, H.; He, K.; Bian, J.; Shao, Y.; Du, J. Transcription factor Sp2 promotes TGFB-mediated interstitial cell osteogenic differentiation in bicuspid aortic valves through a SMAD-dependent pathway. Exp. Cell Res. 2022, 411, 112972.
- Naito, S.; Sequeira-Gross, T.; Petersen, J.; Detlef, I.; Sachse, M.; Zeller, T.; Reichenspurner, H.; Girdauskas, E. Circulating microRNAs in the prediction of BAV aortopathy: Do the expression patterns correlate between blood and aortic tissue? Rev. Cardiovasc. Med. 2022, 23, 47.
- Albinsson, S.; Della Corte, A.; Alajbegovic, A.; Krawczyk, K.K.; Bancone, C.; Galderisi, U.; Cipollaro, M.; De Feo, M.; Forte, A. Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microrna signatures in mildly dilated ascending aorta. Heart Vessel. 2017, 32, 750–767.
- Borghini, A.; Foffa, I.; Pulignani, S.; Vecoli, C.; Ait-Ali, L.; Andreassi, M.G. miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing. Int. J. Mol. Sci. 2017, 18, 2498.
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Aragonès, G.; Faiges, M.; Alegret, J.M. MicroRNAs Clustered within the 14q32 Locus Are Associated with Endothelial Damage and Microparticle Secretion in Bicuspid Aortic Valve Disease. Front. Physiol. 2017, 8, 648.
- Maleki, S.; Cottrill, K.A.; Poujade, F.-A.; Bhattachariya, A.; Bergman, O.; Gådin, J.R.; Simon, N.; Lundströmer, K.; Franco-Cereceda, A.; Björck, H.M.; et al. The mir-200 family regulates key pathogenic events in ascending aortas of individuals with bicuspid aortic valves. J. Intern. Med. 2019, 285, 102–114.
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Baiges, I.; Faiges, M.; Alegret, J.M. Specific circulating microRNA signature of bicuspid aortic valve disease. J. Transl. Med. 2017, 15, 76.
- Gallo, A.; Agnese, V.; Coronnello, C.; Raffa, G.M.; Bellavia, D.; Conaldi, P.G.; Pilato, M.; Pasta, S. On the prospect of serum exosomal miRNA profiling and protein biomarkers for the diagnosis of ascending aortic dilatation in patients with bicuspid and tricuspid aortic valve. Int. J. Cardiol. 2018, 273, 230–236.
- Giusti, B.; Sticchi, E.; De Cario, R.; Magi, A.; Nistri, S.; Pepe, G. Genetic Bases of Bicuspid Aortic Valve: The Contribution of Traditional and High-Throughput Sequencing Approaches on Research and Diagnosis. Front. Physiol. 2017, 8, 612.
- Alonso-Montes, C.; Martín, M.; Martínez-Arias, L.; Coto, E.; Naves-Díaz, M.; Morís, C.; Cannata-Andía, J.B.; Rodríguez, I. Variants in cardiac GATA genes associated with bicuspid aortic valve. Eur. J. Clin. Investig. 2018, 48, e13027.
- Hill, J.C.; Billaud, M.; Richards, T.D.; Kotlarczyk, M.P.; Shiva, S.; Phillippi, J.A.; Gleason, T.G. Layer-specific Nos3 expression and genotypic distribution in bicuspid aortic valve aortopathy. Eur. J. Cardio-Thorac. Surg. 2022, ezac237.
- Wooten, E.C.; Iyer, L.K.; Montefusco, M.C.; Hedgepeth, A.K.; Payne, D.D.; Kapur, N.K.; Housman, D.E.; Mendelsohn, M.E.; Huggins, G.S. Application of gene network analysis techniques identifies AXIN1/PDIA2 and endoglin haplotypes associated with bicuspid aortic valve. PLoS ONE 2010, 5, e8830.
- Fulmer, D.; Toomer, K.; Guo, L.; Moore, K.; Glover, J.; Moore, R.; Stairley, R.; Lobo, G.; Zuo, X.; Dang, Y.; et al. Defects in the Exocyst-Cilia Machinery Cause Bicuspid Aortic Valve Disease and Aortic Stenosis. Circulation 2019, 140, 1331–1341.
- Roberts, W.C. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am. J. Cardiol. 1970, 26, 72–83.
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur. J. Cardiothorac. Surg. 2021, 60, 448–476.
- Brandenburg, R.O.; Tajik, A.J.; Edwards, W.D.; Reeder, G.S.; Shub, C.; Seward, J.B. Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: Echocardiographic-anatomic correlation in 115 patients. Am. J. Cardiol. 1983, 51, 1469–1473.
- Angelini, A.; Ho, S.Y.; Anderson, R.H.; Devine, W.A.; Zuberbuhler, J.R.; Becker, A.E.; Davies, M.J. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. J. Thorac. Cardiovasc. Surg. 1989, 98, 362–367.
- Sabet, H.Y.; Edwards, W.D.; Tazelaar, H.D.; Daly, R.C. Congenitally Bicuspid Aortic Valves: A Surgical Pathology Study of 542 Cases (1991 Through 1996) and a Literature Review of 2,715 Additional Cases. Mayo Clin. Proc. 1999, 74, 14–26.
- Sievers, H.-H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233.
- Schaefer, B.M.; Lewin, M.B.; Stout, K.K.; Gill, E.; Prueitt, A.; Byers, P.H.; Otto, C.M. The bicuspid aortic valve: An integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008, 94, 1634–1638.
- Kang, J.-W.; Song, H.G.; Yang, D.H.; Baek, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Lim, T.-H.; Song, J.-K. Association Between Bicuspid Aortic Valve Phenotype and Patterns of Valvular Dysfunction and Bicuspid Aortopathy: Comprehensive Evaluation Using MDCT and Echocardiography. JACC Cardiovasc. Imaging 2013, 6, 150–161.
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014, 129, 2691–2704.
- Jilaihawi, H.; Chen, M.; Webb, J.; Himbert, D.; Ruiz, C.E.; Rodés-Cabau, J.; Pache, G.; Colombo, A.; Nickenig, G.; Lee, M.; et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc. Imaging 2016, 9, 1145–1158.
- Sun, B.J.; Lee, S.; Jang, J.Y.; Kwon, O.; Bae, J.S.; Lee, J.H.; Kim, D.-H.; Jung, S.-H.; Song, J.-M.; Kang, D.-H.; et al. Performance of a Simplified Dichotomous Phenotypic Classification of Bicuspid Aortic Valve to Predict Type of Valvulopathy and Combined Aortopathy. J. Am. Soc. Echocardiogr. 2017, 30, 1152–1161.
- Murphy, I.G.; Collins, J.; Powell, A.; Markl, M.; McCarthy, P.; Malaisrie, S.C.; Carr, J.C.; Barker, A.J. Comprehensive 4-stage categorization of bicuspid aortic valve leaflet morphology by cardiac MRI in 386 patients. Int. J. Cardiovasc. Imaging 2017, 33, 1213–1221.
- Bernard, C.; Morgant, M.C.; Guillier, D.; Cheynel, N.; Bouchot, O. Point on the Aortic Bicuspid Valve. Life 2022, 12, 518.
- Groenendijk, B.C.; Hierck, B.P.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 2004, 230, 57–68.
- Noiri, E.; Lee, E.; Testa, J.; Quigley, J.; Colflesh, D.; Keese, C.R.; Giaever, I.; Goligorsky, M.S. Podokinesis in endothelial cell migration: Role of nitric oxide. Am. J. Physiol. Physiol. 1998, 274, C236–C244.
- Fernández, B.; Durán, A.C.; Fernández-Gallego, T.; Fernández, M.C.; Such, M.; Arqué, J.M.; Sans-Coma, V. Bicuspid Aortic Valves with Different Spatial Orientations of the Leaflets Are Distinct Etiological Entities. J. Am. Coll. Cardiol. 2009, 54, 2312–2318.
- Fernández, B.; Soto-Navarrete, M.T.; López-García, A.; López-Unzu, M.; Durán, A.C.; Fernández, M.C. Bicuspid Aortic Valve in 2 Model Species and Review of the Literature. Veter. Pathol. 2020, 57, 321–331.
- Phillips, H.M.; Mahendran, P.; Singh, E.; Anderson, R.H.; Chaudhry, B.; Henderson, D.J. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc. Res. 2013, 99, 452–460.
- Sans-Coma, V.; Fernández, B.; Durán, A.C.; Thiene, G.; Arqué, J.M.; Muñoz-Chápuli, R.; Cardo, M. Fusion of valve cushions as a key factor in the formation of congenital bicuspid aortic valves in Syrian hamsters. Anat. Rec. 1996, 244, 490–498.
- Soto-Navarrete, M.T.; López-Unzu, M.; Durán, A.C.; Fernández, B. Embryonic development of bicuspid aortic valves. Prog. Cardiovasc. Dis. 2020, 63, 407–418.
- Evangelista, A.; Gallego, P.; Calvo-Iglesias, F.; Bermejo, J.; Robledo-Carmona, J.; Sánchez, V.; Saura, D.; Arnold, R.; Carro, A.; Maldonado, G.; et al. Anatomical and clinical predictors of valve dysfunction and aortic dilation in bicuspid aortic valve disease. Heart 2018, 104, 566–573.
- Kong, W.K.F.; Delgado, V.; Poh, K.K.; Regeer, M.V.; Ng, A.C.; McCormack, L.; Yeo, T.C.; Shanks, M.; Parent, S.; Enache, R.; et al. Prognostic Implications of Raphe in Bicuspid Aortic Valve Anatomy. JAMA Cardiol. 2017, 2, 285–292.
- Kong, W.K.F.; Regeer, M.V.; Poh, K.K.; Yip, J.W.; van Rosendael, P.; Yeo, T.C.; Tay, E.; Kamperidis, V.; Van Der Velde, E.T.; Mertens, B.; et al. Inter-ethnic differences in valve morphology, valvular dysfunction, and aortopathy between Asian and European patients with bicuspid aortic valve. Eur. Heart J. 2018, 39, 1308–1313.
- Niaz, T.; Poterucha, J.T.; Olson, T.M.; Johnson, J.N.; Craviari, C.; Nienaber, T.; Palfreeman, J.; Cetta, F.; Hagler, D.J. Characteristic Morphologies of the Bicuspid Aortic Valve in Patients with Genetic Syndromes. J. Am. Soc. Echocardiogr. 2018, 31, 194–200.
- Sun, B.J.; Jin, X.; Song, J.-K.; Lee, S.; Lee, J.H.; Park, J.-B.; Lee, S.-P.; Kim, D.-H.; Park, S.-J.; Kim, Y.-J.; et al. Clinical Characteristics of Korean Patients with Bicuspid Aortic Valve Who Underwent Aortic Valve Surgery. Korean Circ. J. 2018, 48, 48–58.
- Yang, L.T.; Pellikka, P.A.; Enriquez-Sarano, M.; Maalouf, J.F.; Scott, C.G.; Michelena, H.I. Stage B Aortic Regurgitation in Bicuspid Aortic Valve: New Observations on Progression Rate and Predictors. JACC Cardiovasc. Imaging 2020, 13, 1442–1445.
- Fernandes, S.M.; Khairy, P.; Sanders, S.P.; Colan, S.D. Bicuspid Aortic Valve Morphology and Interventions in the Young. J. Am. Coll. Cardiol. 2007, 49, 2211–2214.
- Fernandes, S.M.; Sanders, S.P.; Khairy, P.; Jenkins, K.J.; Gauvreau, K.; Lang, P.; Simonds, H.; Colan, S.D. Morphology of bicuspid aortic valve in children and adolescents. J. Am. Coll. Cardiol. 2004, 44, 1648–1651.
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Enriquez-Sarano, M.; Bax, J.J.; Otto, C.M.; Schäfers, H.-J. Speaking a common language: Introduction to a standard terminology for the bicuspid aortic valve and its aortopathy. Prog. Cardiovasc. Dis. 2020, 63, 419–424.
- Sperling, J.S.; Lubat, E. Forme fruste or ‘Incomplete’ bicuspid aortic valves with very small raphes: The prevalence of bicuspid valve and its significance may be underestimated. Int. J. Cardiol. 2015, 184, 1–5.
- American College of Cardiology Foundation Appropriate Use Criteria Task Force; American Society of Echocardiography; American Heart Association; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Critical Care Medicine; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J. Am. Soc. Echocardiogr. 2011, 24, 229–267.
- Guala, A.; Rodriguez-Palomares, J.; Galian-Gay, L.; Teixido-Tura, G.; Johnson, K.M.; Wieben, O.; Avilés, A.S.; Evangelista, A. Partial Aortic Valve Leaflet Fusion Is Related to Deleterious Alteration of Proximal Aorta Hemodynamics. Circulation 2019, 139, 2707–2709.
- Michelena, H.I.; Yang, L.-T.; Enriquez-Sarano, M.; Pochettino, A. The elusive ‘forme fruste’ bicuspid aortic valve: 3D transoesophageal echocardiography to the rescue. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1169.
- Borger, M.A.; Fedak, P.W.; Stephens, E.H.; Gleason, T.G.; Girdauskas, E.; Ikonomidis, J.S.; Khoynezhad, A.; Siu, S.C.; Verma, S.; Hope, M.D.; et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: Full online-only version. J. Thorac. Cardiovasc. Surg. 2018, 156, e41–e74.
- Aicher, D.; Kunihara, T.; Issa, O.A.; Brittner, B.; Gräber, S.; Schäfers, H.-J. Valve Configuration Determines Long-Term Results After Repair of the Bicuspid Aortic Valve. Circulation 2011, 123, 178–185.
- Jahanyar, J.; de Kerchove, L.; El Khoury, G. Bicuspid aortic valve repair: The 180 degrees -Reimplantation technique. Ann. Cardiothorac. Surg. 2022, 11, 473–481.
More
