Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francesco Nappi and Version 2 by Sirius Huang.
Bicuspid aortic valve (BAV) is the most frequent congenital cardiac disease. Alteration of ascending aorta diameter is a consequence of shear stress alterations due to haemodynamic abnormalities developed from inadequate valve cusp coaptation.
- bicuspid aortic valve
- aortopathy
- classification
1. Introduction
Bicuspid aortic valve (BAV) is the most frequent congenital cardiac pathology; has a prevalence of 1–2% [1], a high incidence of adverse outcomes, especially aortic stenosis (AS) and aortic regurgitation (MR) [2]; and is at least three times more common in males than females [3].
Bicuspid aortopathy, reported in 50% of BAV patients, consists of the aorta enlargement starting from the aortic root and involving the aortic arch and depends on blood flux turbulences characterized by power vectors directed against the aortic toot and the convexity of the vessel [4][5][6][7][4,5,6,7]. Recently, micro-RNA (miRNA) has been studied regarding post-transcriptional regulation of genes in aortopathy manifestation. [8][9][8,9].
2. Genetics and Molecular Biology
Estimating mutation genes and their inheritance patterns is challenging [7] because locus 9q34.3 alteration causes mutations in regulators NOTCH1 with secondary pathological aortic valve development [10][11][10,11]; gene damages on 18q, 5q, and 13q induces BAV [12]; and finally, damages to the smooth muscle alfa actine (ACTA 2) gene produce BAV and aortic aneurysms [13]. There is a tight linkage between BAV expression and other congenital pathologies such as the coarctation of the aorta. Concerning BAV phenotype, Shone’s syndrome with a left-sided lesion that can cause inflow and outflow obstruction, Turner’s syndrome with aortic coarctation, and William’s syndrome involving supravalvular stenosis may be observed. Moreover, ventricular septal defect, atrial septal defect, patent ductus arteriosus, and coronary vessels, which may mainly involve single coronary and reversal coronary dominance, have been reported [14][15][16][14,15,16]. Micro-RNAs (MiRNAs) need to be considered in biochemical and molecular changes in BAV and aorthopathy (Table 12). MiRNAs are small, single-stranded, noncoding RNA molecules that determine the post-transcriptional regulation of gene expression. The effects of miRNAs are the result of base pairing with complementary sequences within mRNA molecules that are silenced by cleavage of the mRNA strand, destabilization of the mRNA by shortening its tail, and less efficient translation into proteins by ribosomes [17]. MiRNA expression profiling studies show that the expression levels of certain miRNAs change in diseased human hearts, suggesting their involvement in cardiomyopathies. MiR-712 is a potential predictor of atherosclerosis, has blood flow-dependent expression, and miR-712 is also upregulated in endothelial cells exposed to naturally occurring d-flow in the greater curvature of the aortic arch [18]. Several studies have investigated the cooperation of miRNA, metalloproteinases (MMP), and tissue inhibitor of matrix metalloproteinases (TIMP) in aorthopathy secondary to morphological alteration of the aortic valve. miRNAs related to dilation of the thoracic aorta (TA) are upregulated in transcriptional and epigenetic ways: different levels of MMP-2, MMP-9, TIMP-1, and TIMP-9 were observed [19]. A high level of MMP-2 and increased levels of miR-17 and miRNAs with the same genetic features as miR-17 were found in a comparative study involving patients with mild and severe aorta dilation, with a decreased level of TIMP -1, TIMP-2, and TIMP-3, thus hypothesizing a continuous development of TA influenced by BAV [20]. A recent study showed a relationship between miR-133a and TIMP-1 and TIMP-2 without reporting a statistically significant association between miR-143 and MMP-2 [21].Table 12. Gene expression involved in valve and aortic diseases. ACTA 2: alfa actine 2, AXIN: gene encodes a cytoplasmic protein that contains a regulation of G-protein signaling (RGS) domain and a disheveled and axin (DIX) domain, BAV: bicuspid aortic valve, ENG: Endoglin, FBN1: fibrillin 1, GATA (sequence for transcription factors for zinc proteins’ binding DNA sequence), NOS3: nitric oxide synthase 3, NOTCH1 (gene encoding transmembrane proteins), PDIA2: protein disulfide isomerase family A member 2, PECAM-1: platelet endothelial cell adhesion molecule-1, TGF: transforming growth factor, TIMP: tissue inhibitor of matrix metalloproteinases.
| Gene Expression | Pathology |
|---|---|
| miR-146-5p | BAV, aortic aneuurysm (convex region) |
| miR-21-5p | BAV, aortic aneuurysm (convex region) |
| miR-17 | Aoritc anurysm |
| miR 21 | Aortic aneurysm |
| miR-34 a | Aortic aneurysm |
| miR-122 | BAV |
| miR 130 a | BAV |
| miR-133a | TIMP1,TIMP2, aortic aneurysm |
| mi-R 143 | Aortic aneurysm |
| mi-R 145 | Aortic aneurysm |
| miR 146-5p | Aortic aneurysm |
| miR-200 | Endothelial-mesenchimal/epithelial mesenchimal |
| miR-423-5p | BAV, aortic aneurysm |
| miR-424-3p downregulation | Cell proliferation, apoptosis, endothelial cells alterations, aortic anuerysm |
| miR-486 | BAV |
| miR-494 | PECAM |
| miR-712 | Atherosclerosis, aortic aneurysm |
| miR-718 | Aortic aneurysm |
| ACTA2 | BAV. Aortic aneurysm |
| AXIN1-PDIA2 | BAV |
| ENG | BAV |
| FBN 1 | BAV |
| GATA4/GATA5/GATA6 | BAV |
| NOS3 | BAV |
| NOTCH1 (9q34.3) | BAV, outflow tract malformation |
| TGFb1/TGFb2 | Sporadic BAV, Loeys-Dietz syndrome |
| 18q | BAV |
| 5q | BAV |
| 13q | BAV |
3. Classification and Nomenclature
Since 1970, several classifications of BAV, derived from pathology, US scan, CT scan, and MR patterns (Table 23), have been proposed [36]. Recently, an international consensus statement developed a classification based on the progression of cusps fusion and geometry of commissurae [37], with particular attention to surgical indications and techniques.Table 23. BAV classifications (adapted from Michelena HI et al./European Journal of cardio-thoracic surgery). Abbreviations; BAV, bicuspid aortic valve; BAVCon, bicuspid aortic valve consortium; LN, left non-coronary fusion; RL, right–left fusion; RN, right non-coronary fusion.
| Author | Nomenclature |
|---|---|
| Roberts [36] 1970 | Anterior–posterior cusps Right–left cusps Presence of raphe |
| Brandenburg et al. [38] 1983 | Clock-face nomenclature: Commissures at 4–10 o’clock with raphe at 2 o’clock (R-L) Commissures at 1–6 o’clock with raphe at 10 o’clock (RN) Commissures at 3–9 o’clock without raphe (L-N) |
| Angelini et al. [39] 1989 | Anterior–posterior cusps Right–left cusps Presence of raphe |
| Sabet et al. [40] 1999 | RL RN LN Presence of raphe |
| Sievers and Schmidtke [41] 2007 | Type 0 (no raphe): anteroposterior or lateral cusps (true BAV) Type 1 (1 raphe): R-L, RN, L-N Type 2 (2 raphes): L-R, RN |
| Schaefer et al. [42] 2008 | Type 1: RL Type 2: RN Type 3: LN Presence of raphe Aorta: Type N: normal shape Type E: sinus effacement Type A: ascending aorta dilatation |
| Kang et al. [43] 2013 | Anteroposterior orientation: type 1: R-L with raphe type; 2: R-L without raphe Right–left orientation: Type 3: RN with raphe Type 4: L-N with raphe Type 5: symmetrical cusps with 1 coronary artery originating from each cusp Aorta: Type 0: normal Type 1: dilated root Type 2: dilated ascending aorta Type 3: diffuse involvement of the ascending aorta and arch |
| Michelena et al. [44] 2014 | BAVCon nomenclature: Type 1: R-L Type 2: RN Type 3: L-N Presence of raphe |
| Jilaihawi et al. [45] 2016 | Tricommissural: functional or acquired bicuspidity of a trileaflet valve Bicommissural with raphe Bicommissural without raphe |
| Sun et al. [46] 2017 | Dichotomous nomenclature: R-L Mixed: (RN or L-N) |
| Murphy et al. [47] 2017 | Clock-face nomenclature: Type 0: partial fusion/eccentric leaflet? Type 1: RN, RL, LN partial fusion/eccentric leaflet? Type 2: RL and RN, RL and LN, RN and LN partial fusion/eccentric leaflet? |
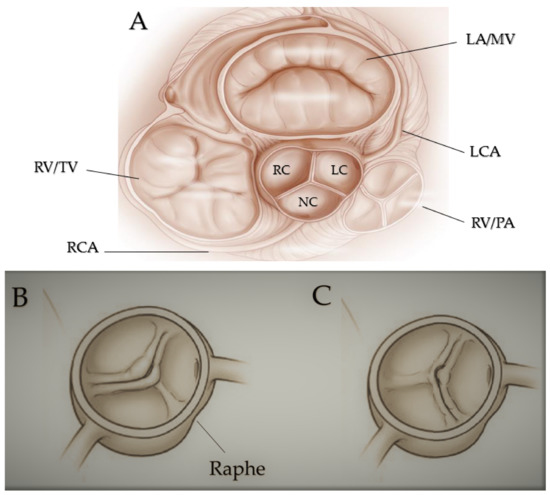
Figure 1. Fused bicuspid aortic valve. (A) Represents short-axis normal tricuspidal aortic pattern with anatomical proximities. Cusps’ fusion patterns seen in short heart axis: right-left coronary fusion (B), right-non coronary fusion (C). All BAVs have three sinuses. Raphe structure is between the fused cusps. Non-fused cusp is prominent in respect to the fused ones. The commissure angle of the non-fused cusp has a degree < 180°. Abbreviations: LA, left atrium; LC, left cusp; LCA, left coronary artery; MV, mitral valve; NC, non-coronary cusp; PA, pulmonary artery; RA, right atrium; RC, right cusp; RCA, right coronary artery; RV, right ventricule; TV, tricuspidalic valve. Licenses Centre Cardiologique du Nord; order date 8 September 2022; order number 5384080341542; publication NEJM; Title: Mitral valve Repair for Mitral valve prolapse.
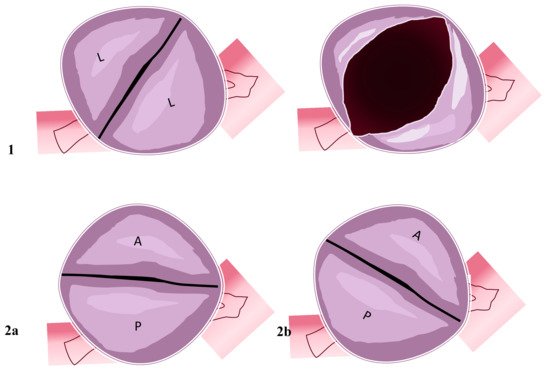
Figure 2. Two-sinus bicuspid aortic valve. Figure represents two cusps’ non-fusion patterns seen in the short heart axis. Aortic valves have two sinuses with two leaflets non-derived from fusion mechanisms. (1) Coronary arteries originate from the two sinuses with two lateral leaflets. The opened valve in systole phase has the oval-ball image. (2a) In this position, coronary arteries originate from the anterior sinus (right coronary artery) and posterior sinus (common left stem). (2b) Right coronary artery and common left stem both originate from anterior sinus. Abbreviations; A, anterior; L, lateral P, posterior.
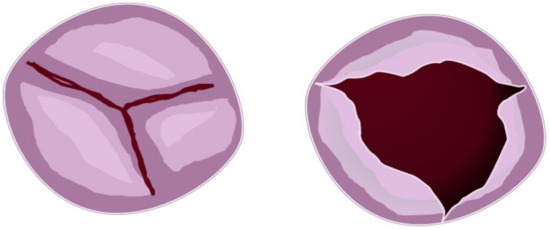
Figure 3. Partial fusion aortic valve. Figure represents three cusps with partial leaflets fusion seen in the short heart axis (left). In this case, the opened (right) aortic valve is similar to the normal valve but with a more narrow area.
4. BAV Geometry Types and Surgical Implications
In every subtype of the previous classification, we can identify the BAV geometrical pattern by evaluating the position of commissures related to the aortic orifice, their angle in the coaptation zone, the presence of raphe, and the morphology and the area of the cusps. In the fused BAV type, it is relevant to establish the relationships between the fused cusps and non-fused cusp and the angle of the commissures of the non-fused cusps (Figure 4).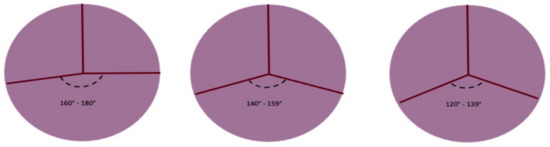
Figure 4. Symmetry of fused bicuspid aortic valve (adapted from Michelena HI et al./European Journal of cardio-thoracic surgery). Figure represents the angles determined by aortic valve leaflets fusion patterns. The length of raphe causes the retraction of fused leaflets and the non-physiological coaptation of the non-fused leaflet with fused leaflets. The geometry of the three patterns can be summarized as symmetrical, asymmetrical, and very asymmetrical The degree of the angle is important for surgical technique.
