Liver resection for malignant tumors should respect oncological margins while ensuring safety and improving the quality of life, therefore tumor staging, underlying liver disease and performance status should all be attentively assessed in the decision process. The concept of parenchyma-sparing liver surgery is nowadays used as an alternative to major hepatectomies to address deeply located lesions with intricate topography by means of complex multiplanar parenchyma-sparing liver resections, preferably under the guidance of intraoperative ultrasound. Regenerative liver surgery evolved as a liver growth induction method to increase resectability by stimulating the hypertrophy of the parenchyma intended to remain after resection (referred to as future liver remnant), achievable by portal vein embolization and liver venous deprivation as interventional approaches, and portal vein ligation and associating liver partition and portal vein ligation for staged hepatectomy as surgical techniques. Interestingly, although both strategies have the same conceptual origin, they eventually became caught in the never-ending parenchyma-sparing liver surgery vs. regenerative liver surgery debate. However, these strategies are both valid and must both be mastered and used to increase resectability.
- liver resection
- therapeutic options in liver surgery
- regenerative liver surgery
- parenchyma- sparing liver resection
1. Parenchyma-Sparing Liver Surgery
- -
-
systematic extended right posterior sectionectomy, as an alternative to right hemi-hepatectomy [23][24]—segment (S) 6–7 resection partially extended to S5 and/or S8 with right HV division; middle HV branches supply outflow of preserved S5 and/or S8;
- -
-
mini-upper transversal hepatectomy, as an alternative to right hemi-hepatectomy—S7–8 anatomic or limited resection with right HV division; inferior right HV [24][25], middle HV branches or distal CVs between right and middle HVs supply outflow of S5–6 [7][10];
- -
-
right upper transversal hepatectomy, as an alternative to right extended hemi-hepatectomy [25][26]—S7–S8–S4 superior anatomic or limited resection with right and middle HV division; inferior right HV and/or distal CVs between right, middle and left HVs supply outflow of S4 inferior–5–6;
- -
-
left upper transversal hepatectomy, as an alternative to left extended hemi-hepatectomy [7][10]—S2–S4 superior or S2–S4 superior–S8 anatomic or limited resection with left HV or left and middle HV division; distal CVs between left, middle and/or right HV supply outflow of S3–4 inferior–5;
- -
-
total upper transversal hepatectomy [7][10]—S2–S4 superior–S7–S8 anatomic or limited resection with right, middle and left HV division given the existence of an inferior right HV and CVs between hepatic HVs stumps, that provide outflow of S3–S4 inferior–S5–S6;
- -
-
mini-mesohepatectomy, as an alternative to central hepatectomy [26][27]—S4 superior–S8 anatomic or limited resection with middle HV division; distal CVs between middle HV and right and left HVs supply outflow of S5–S4 inferior;
- -
-
liver tunnel, as an alternative to central hepatectomy plus S1 segmentectomy [7][27][10,28]—S8 anatomic or limited resection with complete S1 removal;
- -
-
liver tunnel extended to segment 4 superior, as an alternative to central hepatectomy plus S1 segmentectomy [7][27][10,28]—S4 superior–S8 anatomic or limited resection with complete S1 removal and middle HV division; distal CVs between middle HV and right and left HVs supply outflow for S5–S4 inferior;
- -
-
systematic limited central, as an alternative to central hepatectomy—sparing the portal pedicle (P) for S8 dorsal and some of P4 and/or P5 pedicles (depending on tumor location). IOUS guides the right transection plane along the P8 dorsal, intersecting the P8 ventral and as few P5 pedicles as possible. The left transection plane is settled relative to tumor position between Cantlie’s line and the falciform ligament [28][29];
- -
-
left anterior sectorectomy, as an alternative to left hepatectomy for lesions invading the distal part of the umbilical portion of the left portal vein–resection of S3 and S4 inferior, while preserving the P2 and P4 superior.
2. Regenerative Liver Surgery
Regenerative surgery (RS) evolved as a method to increase resectability by stimulating the hypertrophy of the parenchyma intended to remain after resection, which is referred to as future liver remnant (FLR) [29][30]. This liver growth induction can be achieved by portal vein embolization (PVE) and liver venous deprivation (LVD) as interventional approaches, and portal vein ligation (PVL) and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) as surgical techniques.
PVE was conceived by Makuuchi et al. in 1990 as a tool to induce hypertrophy of the FLR and decrease the risk of liver failure after major hepatectomy, enabling major anatomical LR, which would otherwise not be feasible [30][31]. The mechanism behind this approach is based on the redirection of the portal flow, that stimulates the contralateral hypertrophy. PVE is associated with a low morbidity and mortality. However, the growth of FLR is limited to a volume by 40% at best, for most cases within a period of around 2 months [31][32]. This may lead to insufficient FLR and/or tumor progression while waiting for hypertrophy. PVL (open or minimally invasive surgery) is a feasible alternative to PVE. For patients undergoing PVE, major hepatectomy becomes feasible in 2/3 of cases with a similar overall survival to those without PVE [32][33]. Chemotherapy after PVE decreases the tumor progression rate and has not been shown to decrease liver hypertrophy. In about 1/3 of patients, PVE fails and leads to canceling of the planned LR (drop-out rate) [33][34].
Recently, liver venous deprivation (LVD), consisting of embolization of both the PV and one or two HVs of the hemi-liver, has been proposed as a promising way for improved regeneration (1–2 weeks) [35,36]. Several studies comparing LVD to PVE reported improved FLR volume growth following LVD [34][35][36][37,38,39], as well as better FLR functional regeneration [37][40]. In particular, one study has shown a more than 75% increase in the kinetic growth rate of the FLR after LVD compared to PVE [35]. Moreover, a 54% functional increase in the FLR 7 days after LVD has been reported [37][40]. However, literature data on LVD of a cirrhotic liver are lacking [38][41].
The two-stage hepatectomy (TSH) was introduced in 2000 as two successive surgical steps for removing multiple bilobar tumors that cannot be removed by a sole hepatectomy [39][42]. Usually, the response to neoadjuvant chemotherapy was used to select candi- dates with favorable tumor biology. TSH can be used by itself or combined with PVE or portal vein ligation (PVL) [40][43]. It usually has resection rates of up to 70–75%; the main reason for non-completion is disease progression between the two stages (around 90% of cases) [39][41][42,44]. The postoperative morbidity rate is around 20% after the 1st stage and 40% after the 2nd stage, with an overall mortality below 5% [42][45].
In 2012, Schnitzbauer et al. proposed combining PVL with in situ liver partition to obtain rapid FLR hypertrophy (in 7–10 days) as a new strategy to increase resectability [43][46], which was subsequently termed ALPPS [44][47]. One mechanism behind this technique is thought to trigger an inflammatory response that induces a growth rate of 22–35 mL daily, significantly superior to PVE (3–5 mL daily) [45][48]. However, this volume growth does not automatically equal an increase in liver function [46][49]. This strategy results in a FLR increase of up to 80% and above (compared to 40% in PVE/PVL), while shortening the interstage period to 1–2 weeks [47][50]. Moreover, ALPPS enables resection rates to increase to more than 90% [48][49][50][51,52,53], now being feasible even when using a minimally invasive approach [51][54].
- -
- -
- -
- -
- -
- -
Of note, although relatively easy to perform, tourniquet ALPPS [64] mighortt be as- sociated with a higher risk of operative events during the second stage due to severe adhesions/perihilar fibrosis.
Short-term results after ALPPS, that were initially a major concern, have been con- tinuously improved over time, now reaching 90-day mortality rates below 5% [61][65] and a relatively low major morbidity (21%) in high-volume centers.
To further increase resectability while reducing morbidity, scholarswe proposed a new techni- cal variant of ALPPS—parenchyma-sparing ALPPS (psALPPS)—that involves shifting the transection plane through segment 4 using IOUS guidance, preserving part of this segment along with the left lateral section [66]. Besides avoiding S4 necrosis, that is a source of complication when performing conventional ALPPS, a significant advantage of psALPPS lies in preventing major bile leaks at the transection surface by avoiding complete exclusion of S4 from the biliary system (as in conventional ALPPS). Parenchyma-sparing ALPPS offers the advantage of maximizing FLR while simultaneously reducing ischemic injury of S4 compared to conventional ALPPS (Figures 1 and 2). Moreover, when compared to stan- dard ALPPS, partitioning through segment 4, away from the umbilical portion of the left portal pedicle, protects against potential injuries of the vascular and biliary structures for segments 2 and 3. This new technical variant also embeds some of the main modifications already proposed, such as partial ALPPS, avoiding the transection beyond the middle HV, and delayed ALPSS [62][66]. It also adapts the concept of avoiding hilar dissection by adopt- ing a minimal hilar dissection (right side approach only) [62][66]. Using an extra-Glissonean approach to complete the hepatectomy during the second step further increases safety by avoiding re-dissection of the liver hilum.
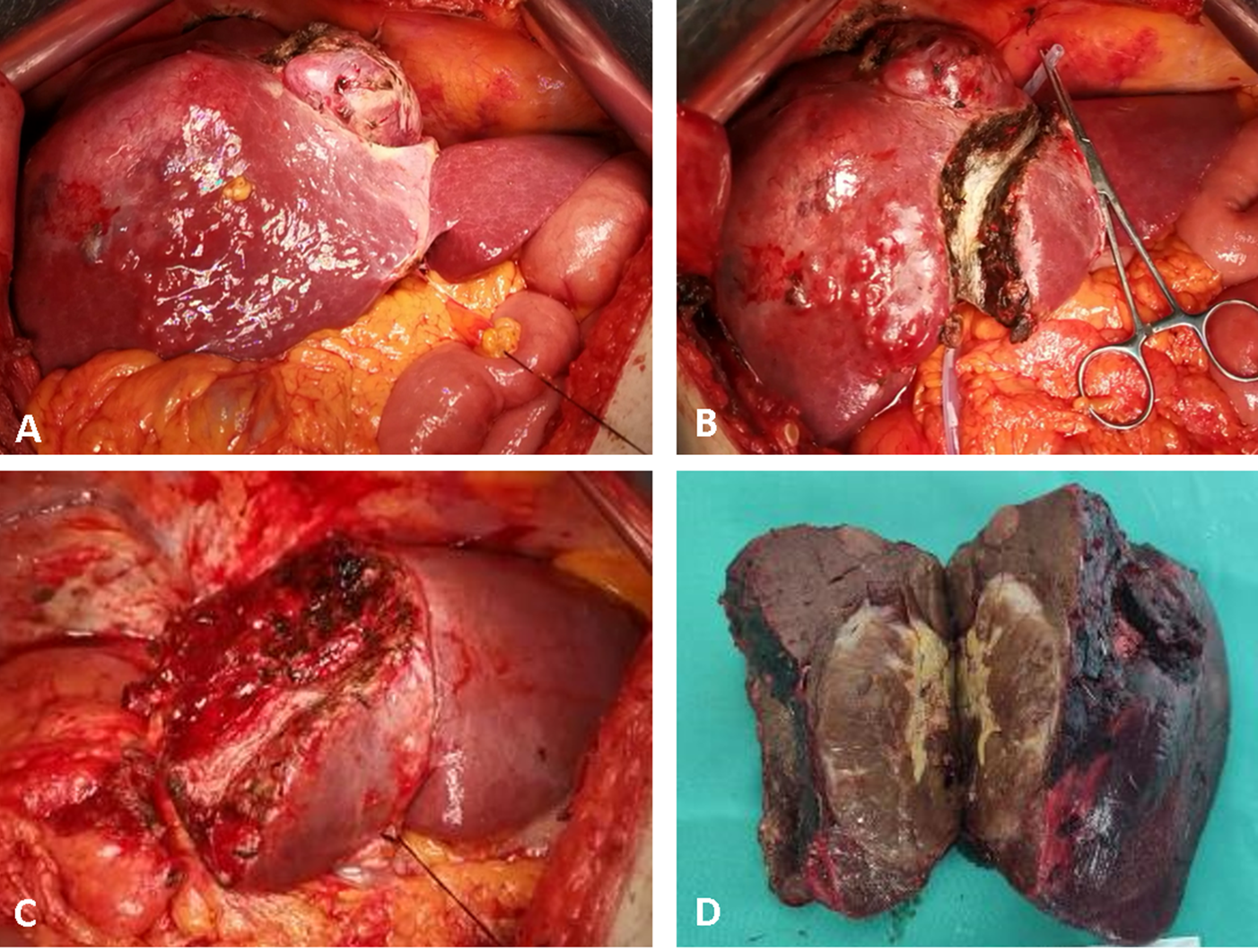
Figure 1. Intraop
Beratsive aspects of parenchymal sparing ALPPS in a 67-year-old male pades being tihent, for a large HCC located in segments 4, 5, 6, 7 and 8, with satellites in segment 4, on HBV chronic hepatitis. Stage 1: (A) intra best available tool foperative aspect at exploration; (B) ultrasoun id-guided partitioning of the liver through segment 4, adding the non-tumoral parenchyma of segment 4 to the FLR. Stage 2 after an interstage interval of 14 days; (C) remnant ntifying and mapping the focal liver after completion of right hemi-hepatectomy non-anatomically extended to segment 4; (D) surgsions in real tical specimen. No intraoperative adverse events were encountered during both operations, and only minor ascites after stage 2 were recorded as complications.
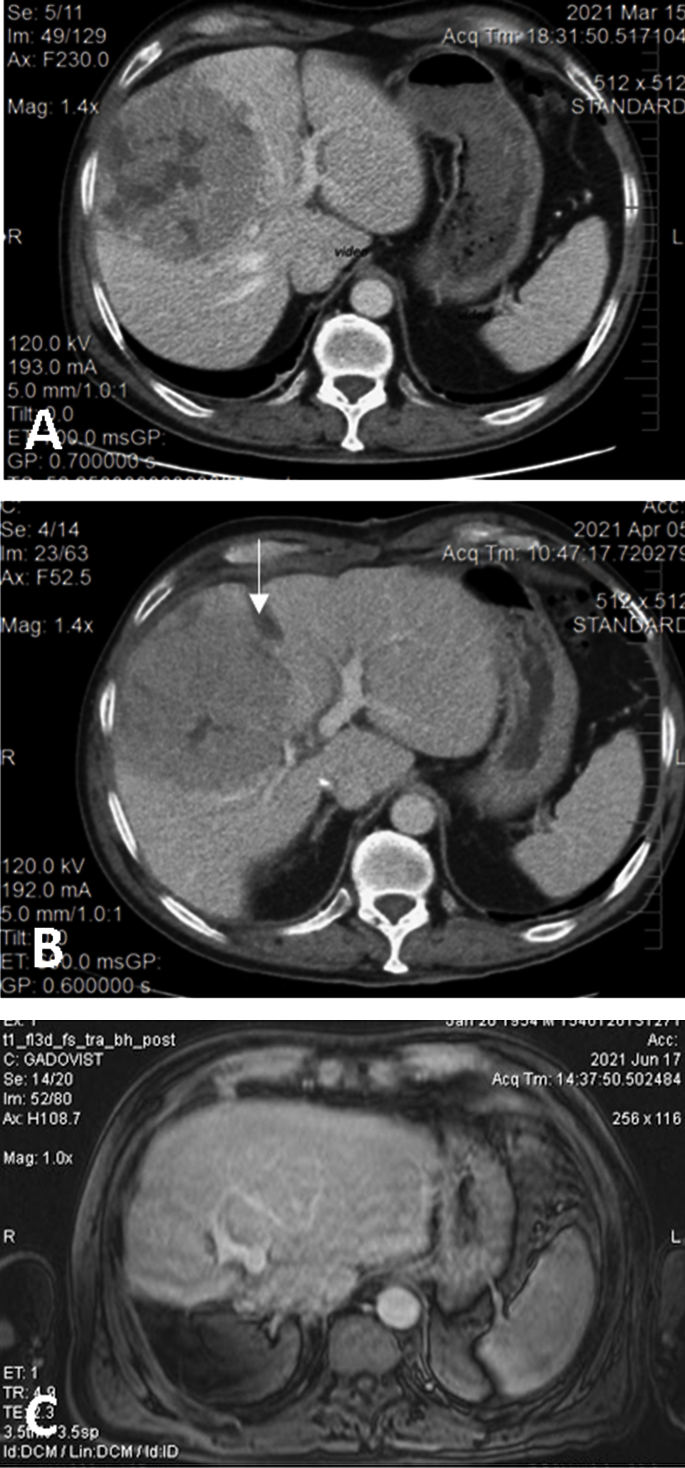
Figure 2., IOUS increases the safety of ALPPS and its variants (A).by Prideoperative CT showntifying the large HCC loanatomic
vated rin segments 5 and 8 with extension to segments 6, 7, 4, compressing the middle hepatic vein; volumetry: volume of segments 2 and 3, 16.8% of total functional liver volume, volume of FLR 27.8%. (B) Interstage CT showants of the portal veing the liver partitioning, absence of contrast inbifurcation and guiding the right portal vein (due to ligation), and sufficient growth of FLR (38.5% of total functional liver volume). (C) Pos and the liver toperative CT with well-perfused, non-dilated bile ducts, and tumor-free remnant livernsection.
3. Parenchyma-Sparing vs. Regenerative Liver Surgery
3. Parenchyma-Sparing vs. Regenerative Liver Surgery
PsALPPS combines both concepts of complex liver surgery, RS and PSS, which syn- ergistically achieve resectability, which would otherwise not be possible with either approach [66].
The minimally invasive approach is feasible for both PSS and RS. However, for com- plex bilobar deeply located liver tumors, PSS is often not feasible due to technical limitations of this approach, making the RS approach, even ALPPS, a technical alternative. Nevertheless, the type of approach should not change the indication for a certain resection strategy. Therefore, if complex PSS is indicated, this should be carried out even if it is feasible only by open approach, and not switched to major two-stage LR only to perform laparoscopic surgery.
3.1. Colorectal Liver Metastases
Downstaging of initially unresectable CRLM may be achieved using novel cytotoxic and biologic systemic therapy to achieve curative surgery with best results when carried out in tertiary referral centers with an expert multidisciplinary team [64][65][68,69]. Compared with non-PSS, perioperative outcomes are better in the case of PSS, with PSS also being associated with satisfactory oncological results. By sparing liver parenchyma, PSS allows repeat hepatectomy in the likely event of liver recurrence. PSS also showed beneficial OS and RFS rates [9][11]. Matsumura et al. showed that, in advanced CRLM, PS LR was not associated with more positive surgical margins or local recurrence when compared with major LR [66][70]. Mise et al. showed that PS LR impacts OS, RFS or liver-only RFS by allowing repeat LR, with similar perioperative morbimortality [67][71], while not increasing the local recurrence risk [16][17]. Neither survival, recurrence risk or site are influenced by the extent of a negative surgical margin [68][72]. In the setting of modern chemotherapy, Adam et al. demonstrated that R1 margins may yet be linked with similar OS [69][73]. The R1vasc approach is safe concerning oncological results in CRLM [70][74], yet hepatectomy en bloc with a vascular element is the preferable approach, given the confirmed true vascular invasion [71][18]. When compared to major LR, PS LR was linked to lower overall morbidity, fewer major complications and a shorter hospital stay while no significant differences were observed for postoperative liver insufficiency and positive resection margins [72][75]. The tumor burden (with a score ≥4.5) is related to a higher rate of positive resection margins both in major and PS LR [72][75]. Independently of tumor burden, the 5-year OS and RFS were similar for PS LR [72][75]. RS, such as PVE [73][74][76,77] and TSH [43][75][46,78], has made it possible for more patients with colorectal liver metastases (CRLMs) to benefit from curative LR [76][77][79,80]. Unresectable CRLMs are often related to a difficulty in completely removing all lesions and preserving as FLR at least two contiguous functional segments [78][81].3.2. Hepatocellular Carcinoma
There are several curative options for the treatment of hepatocellular carcinoma (HCC), e.g., usually intraoperative or percutaneous tumor ablation (for tumors <3 cm), hepatectomy and liver transplantation, particularly in the setting of advanced cirrhosis [79][89]. Targets of LR are improving recovery, reducing postoperative morbidity and ensuring a satisfactory function of a frequently cirrhotic liver [79][89]. R0 resection is no longer considered an absolute requirement, as R1 resection has become an accepted option for encapsulated HCC in contact with major vascular and biliary structures [80][90]. Hasegawa et al. proved that anatomical LR is oncologically superior to non-anatomical LR [81][91]. As anatomical LR included segmentectomies and sub-segmentectomies [81][91], itwe underlined that this strategy is also a parenchyma-sparing one. However, other studies showed no differences regarding oncological outcomes between anatomical and non-anatomical LR [82][83][84][92,93,94]. PS LRs are especially beneficial in case of cirrhosis. When compared to the right posterior sectionectomy, the right hepatectomy for HCC was more frequently associated with liver failure (9.4% vs. 2%), yet with similar 5-year OS (83% vs. 76%) and RFS (52% for both) [85][95]. Therefore, the right posterior sectionectomy is to be selected over the right hepatectomy in cases that allow complete tumor resection [79][89]. When the right posterior sectionectomy cannot ensure resectability, the systematic extended right posterior sectionectomy (SERPS) has been proved to be a feasible alternative to the right hepatectomy [23][24]. Liver failure risk and mortality are also increased due to an insufficient FLR in the setting of cirrhosis in patients with central tumors, for which extended right or left hepatectomy has been the standard recommended approach. An alternative is central or mesohepatectomy (S4–5–8 and middle HV resection), that preserves more parenchyma while ensuring complete tumor resection [79][89]. It has been shown that following a central hepatectomy, postoperative bilirubin levels >4 mg/dL are notably less common (2% vs. 39%) [86][96] and the liver failure risk is lower (1.7% vs. 10.6%) [87][97] than after an extended right/left hepatectomy; yet, the 5-year OS and RFS are similar [87][97]. As it is still a major hepatectomy, central hepatectomy is, however, to be avoided whenever possible. A feasible alternative is the systematic limited central hepatectomy [28][29]. The current practice involves performing parenchyma sparing, preferably anatomic LR, such as sub-segmentectomies, segmentectomies, bisegmentectomies, right posterior sectionectomy or central hepatectomy [79][89]. In case this is not feasible, non-anatomic LR such as SERPS [23][24] or systematic limited central hepatectomy [28][29] are options to be deployed whenever possible to avoid a major hepatectomy.3.3. Intrahepatic Cholangiocarcinoma
4.3. Intrahepatic Cholangiocarcinoma
Anatomic and non-anatomic LRs are associated with comparable intraoperative bleeding and morbidity, but liver failure occurs more often following anatomic LR [88][111]. Non-anatomic LR has been linked with a higher rate of positive surgical margins, but this seemed to not impact the OS or the DFS [88][111]. However, it has also been shown that negative surgical margins are associated with a beneficial OS and PFS following resection for ICC [89][112]. Positive margins have been linked to inferior results in the long run and the OS and DFS proved to become gradually worse for a margin width >1 cm [90][113]. In this sense, R1 vascular resection is not recommended [91][114]. Non-anatomic LR has proved to be non-inferior to anatomic LR in terms of survival in the case of solitary ICC not invading contiguous organs or extrahepatic metastases, which shows that these patients, particularly in the context of cirrhosis, could benefit more from a non-anatomic hepatectomy, given the lower risk of liver failure [88][111].3.4. Hilar Cholangiocarcinoma
Resectability of hilar cholangiocarcinoma is mainly dictated by the intrahepatic tumor extension. Bismuth–Corlette type III tumors are usually resectable by performing a hemi-hepatectomy that can be extended, while Bismuth–Corlette type IV tumors are surgically manageable only in selected patients. Other aspects that determine resectability are the tumor invasion of the portal vein and/or hepatic artery, FLR in terms of both volume and functional status as well as its competent biliary drainage and PVE feasibility. Regardless of resection extent, en bloc S1 resection is advised [92][117]. Left liver resections are more beneficial as they allow sparing the right liver, hence left trisectionectomy is a feasible option for cases of Klatskin IV tumors that do not involve the right hepatic artery [92][117]. Mesohepatectomy should be taken into consideration when the bile ducts of S6–7 and S2–3 are not affected, and the portal and arterial branches of these sparable segments can be preserved [92][117]. Strategies to maximize liver functional status using PSS to reduce the extent of LR in Klatskin tumor have resulted in a lower overall mortality [92].3.5. Other Focal Liver Lesions
Indications for surgical resection of GIST include limited disease, progression refrac- tory to TKI and locally advanced or previously unresectable tumors that manifest favorable response to neoadjuvant therapy with TKI [93]. In the case of NELM, LR is the treatment of choice whenever feasible, since patient outcomes after resection have been reported to be favorable compared to those with unresectable tumors [120117]. Repeat hepatectomy, if feasible, can be a good option for intra- hepatic recurrence and can provide long-term survival [94]. Regarding hemangiomas, as complications are rare, observation is justified in the absence of symptoms. LR is indicated in patients with abdominal (mechanical) complaints or complications or when diagnosis remains inconclusive. Enucleation is the preferred surgical method according to existing literature [95]. ALPPS may be also deployed in neuroendocrine liver metastases (NELMs) [96], and other rare indications, such as lymphoma [97]. In NELM, 2-year overall survival rates of 95.2% were reported [96].3.5. Other Focal Liver Lesions
Indications for surgical resection of GIST include limited disease, progression refrac- tory to TKI and locally advanced or previously unresectable tumors that manifest favorable response to neoadjuvant therapy with TKI [119].
In the case of NELM, LR is the treatment of choice whenever feasible, since patient outcomes after resection have been reported to be favorable compared to those with unresectable tumors [120]. Repeat hepatectomy, if feasible, can be a good option for intra- hepatic recurrence and can provide long-term survival [121].
Regarding hemangiomas, as complications are rare, observation is justified in the absence of symptoms. LR is indicated in patients with abdominal (mechanical) complaints or complications or when diagnosis remains inconclusive. Enucleation is the preferred surgical method according to existing literature [122].
ALPPS may be also deployed in neuroendocrine liver metastases (NELMs) [123], and other rare indications, such as lymphoma [124]. In NELM, 2-year overall survival rates of 95.2% were reported [123].
