
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fulvio Nisi | + 3752 word(s) | 3752 | 2021-05-11 10:47:57 | | | |
| 2 | Karina Chen | -7 word(s) | 3745 | 2021-05-24 05:35:04 | | | | |
| 3 | Karina Chen | -7 word(s) | 3745 | 2021-05-24 05:37:03 | | |
Video Upload Options
Major high-risk surgery (HRS) exposes patients to potential perioperative adverse events. Hepatic resection of colorectal metastases can surely be included into the HRS class of operations. Limiting such risks is the main target of the perioperative medicine.
1. Preoperative Considerations
Preoperative assessment was adapted to individual patients and types of surgical resection. Young patients (<40 years) without underlying liver disease could undergo significant liver resection having the same preoperative work out of any major intra-abdominal operation. Conversely, patients with hepatic disease have an increased risk of intra- and postoperative complications and require an in-depth preoperative assessment.
During the past decades, the liver surgery-associated mortality has reduced to less than 2% in referral centers, but the rate of postoperative adverse events is still high (20–50%) [1][2][3]. To quantify the operation-related risk, many score systems have been used.
Despite the ASA (American Society of Anesthesiology physical status classification system) score being a simple tool adopted all over the world to evaluate patients preoperatively, it does not take into account the type of operation the patient undergoes. In 2014, the European Society of Cardiology/European Society of Anesthesiology (ESC/ESA) guidelines on non-cardiac surgery recommended a healthy lifestyle and the correction of unstable clinical conditions to make patients arrive to the surgical theater with a sufficient functional capacity [4][5].
The DASI (Duke Activity Status Index) should be a reliable system of evaluating and predicting the postoperative outcome, in particular, adverse cardiac events [6]. Finally, the surgical Apgar score (SAS), even though it is not so widely used, could be a complementary reliable and simple system for estimating the risk of a poor postoperative outcome [7].
At any rate, the preoperative period should ameliorate the starting clinical conditions of patients, aiming at better results of the perioperative period.
The ERAS Society recently released guidelines for fast-track management of patients undergoing liver surgery. The recommendations for the perioperative management of patients could be summarized as described below. (1) Recommended preoperative fasting of 6 h for solids and 2 h for liquids. Carbohydrate supplies may be used the evening before surgery and at least 2 h before anesthesia induction. (2) Short-acting anxiolytic drugs should be preferred over the long-acting ones to facilitate regional anesthesia prior to the surgery. (3) Administration of low-molecular-weight heparin (LMWH) or unfractionated heparin should start 2–12 h before the surgery aiming at reducing the risk of thromboembolism. (4) Minimally invasive procedures should be preferred where possible. (5) Maintenance of intraoperative normothermia. (6) Early oral intake at the first postoperative day and early mobilization (as soon as possible). (7) Routine epidural analgesia (EA) cannot be recommended for open liver surgery for ERAS patients. Wound infusion catheter or intrathecal opiates can be good alternatives combined with multimodal analgesia. (8) Prevention of postoperative nausea and vomiting (PONV). (9) Fluid management includes the central venous pressure (CVP) guide with a target < 5 cm H2O. (10) Steroids (methylprednisolone) may be used before hepatectomy in normal liver parenchyma since it decreases liver injury and intraoperative stress without increasing the risk of complications. (11) Glycaemia control [8].
Cardiac function evaluation should assess the ability of the system to cope with the hemodynamic challenge of vascular exclusion during liver resection. Exercise or stress echocardiography may be useful to assess the contractile reserve. Relevant anamnestic data are those regarding previous neo-adjuvant chemotherapy, which may reduce functional cardiac reserve, and/or conditions causing elevation of central venous pressure (CVP) that significantly increase the risk of intraoperative bleeding [9].
Pulmonary evaluation focuses on detecting impaired pulmonary gas exchange or inadequate ventilatory reserve. Pulmonary function testing and arterial blood gas analysis should be considered in case of abnormal values at pulse oximetry testing. A significant percentage of patients with cirrhosis or portal hypertension is affected by some degree of hepatopulmonary syndrome, producing varying degrees of hypoxemia depending on the degree of intrapulmonary shunting. Typical signs are platypnea (upright positional dyspnea), finger clubbing and spider nevi. In such cases, contrast-enhanced echocardiography is recommended for diagnosis, even if it cannot quantify the extent of shunting.
Hepatic reserve quantification is helpful in predicting the risk of postoperative liver failure. Currently, no single test reliably predicts postoperative liver failure, and the assessment is based on laboratory and radiological investigations, quantitative tests, and surgical judgment. Conventionally, in young patients (<40 yrs) with normal hepatic parenchyma, it is safe to remove up to 50–60% of the whole volume of the liver, whilst patients with chronic liver disease presenting for liver resection are at high risk of postoperative liver failure and require a more detailed assessment of the hepatic function. Indeed, cirrhosis limits the ability of the liver to regenerate. Finally, we should remember that it is contraindicated to operate patients presenting with obstructive jaundice or for emergency liver resection since these factors associated with the highest perioperative morbidity and mortality [10][11].
The Child–Pugh clinical scoring system has been used as a reliable validated prognostic tool for patients with chronic liver disease undergoing general or portocaval shunt surgery and has gained widespread use in hepatobiliary surgery, mostly in case of Hepatocellular carcinoma (HCC). It has recently been suggested that patients with scores of B or C should not receive liver resection surgery [12]. Further specialized testing of the hepatic function, such as assessment of indocyanine green (ICG) retention, is available, but its description goes beyond the scope of this article.
2. General Anesthesia Management and Intraoperative Hemodynamics
The anesthetic issues for this type of surgery are almost the same as for other major operations. General anesthesia is mandatory and short-acting drugs should be preferred. Needless to say, communication regarding surgical manipulations and management of hemodynamics between the anesthesiology and surgical staff is a critical component of achieving the optimal outcome.
Blood flows to the liver (around 1.5 L/min) through two vascular systems: 20% flows from the hepatic artery (HA), 80%—from the portal vein (PV). This amount of blood returns to the right side of the heart by suprahepatic veins that merge into the inferior vena cava just before it flows into the right atrium. Several blood loss-limiting surgical techniques could be adopted by the surgical team aiming at reducing blood loss, hemodynamic impairment, and transfusion-related complications. Hence, excluding hepatic circulation from systemic circulation during dissection and transection of parenchyma might be achieved by means of temporary hepatic inflow occlusion (Pringle maneuver) and total (inflow and outflow) vascular exclusion (TVE).
The Pringle maneuver (PM) consists of intermittent clamping of the hepatic hilum (HH): generally, 15–20 min of interrupted blood flow are followed by 5 min of reperfusion. The PM has been shown to provide a sort of protection of the liver tissue from hepatic injury due to the ischemia-reperfusion injury (I-RI) which should be inevitable for longer and non-intermittent occlusion of the blood flow, [9].
The most effective vascular control is obtained with total vascular exclusion (TVE). After performing the Pringle maneuver, clamps are applied sequentially across the infra-hepatic IVC above the renal veins and across the suprahepatic IVC.
Intuitively, with this technique, hemodynamic instability is likely and potentially profound, with venous return decreasing and systemic vascular resistance increasing, and requires aggressive hemodynamic management [11].
At our hospital, the PM is almost always chosen as the main option. The intermittent interruption of the blood flow makes the cardiac afterload and preload vary coherently. After the HH clamping, the afterload can increase by 20–30% and cardiac output (CO) could fall up to 10%. The PM also aims at limiting the blood loss. When necessary, the ultrasound-guided finger compression of the right hepatic vein by the surgeon increases the effectiveness of the PM to control backflow bleeding [13].
Although evidence of the clinical benefit of administration of N-acetylcysteine (NAC) to limit the I-RI remains contradictory, several studies have reported the effectiveness of NAC (15 mg/kg i.v. bolus) to help cells to metabolize free radicals that are released during the ischemic phase [14][15][16][17][18].
Given the above, it is of utmost importance to adopt hemodynamic monitoring during the surgery (Figure 1).
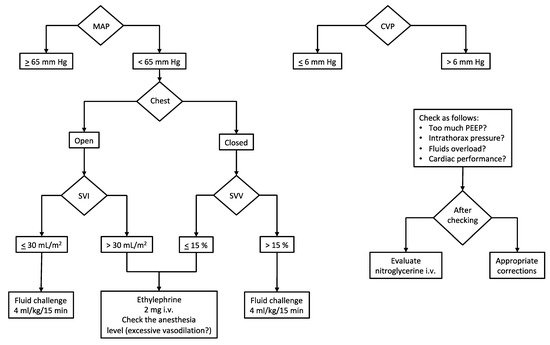
Figure 1. Intraoperative hemodynamic management. Our institutional algorithm for intraoperative hemodynamic management based on target MAP and CVP. Preload dynamic indices (or stroke volume indices) are used to assess fluid responsiveness and guide vasopressor administration. Abbreviations and units. CVP, central venous pressure (mm Hg); MAP, mean arterial pressure (mm Hg); PEEP, positive end-expiratory pressure (cm H2O); SVI, stroke volume index (mL/m2); SVV, stroke volume variation (%).
Beyond the standard monitoring (invasive blood pressure, IPB; electrocardiogram with heart rate, HR; end-tidal CO2, EtCO2; peripheral oxygen saturation, SpO2), the most important parameters which deserve to be measured are cardiac output (CO), stroke volume (SV) and stroke volume variation (SVV) when open-chest surgery is not needed. Since the SVV depends on the interaction between the heart and the lungs (i.e., intrathoracic pressures), this parameter is not reliable in case of the open chest. Conversely, when the chest is closed, the intermittent rising of the intrathoracic pressure (due to positive pressure ventilation) limits the venous return and, consequently, the cardiac stroke volume cyclically. Such continuous variation of the SV is called stroke volume variation and is expressed as a percentage. When SVV is higher than 13–15%, it means that the SV and the CO can be increased by the fluid load (i.e., fluid responsiveness) [19].
Safe cardiocirculatory assessment considers target hemodynamic values as follows: CVP, 0–6 mm Hg; CI, 2–3 L/min/m2 of the body surface area (provided the stroke volume index > 30 mL/m2 of the body surface area in subjects with normal cardiac ejection fraction, >55%); SVV, 10–15% during the resection phase (a value of SVV ≤ 10% could be accepted once liver dissection has concluded).
A peculiar issue regards the monitoring of central venous pressure (CVP). There is a general agreement on its maintenance below 6 mm Hg to limit the backflow bleeding due to the suprahepatic veins’ increased resistance in case of high pressure into the right atrium during the resection phase. Hence, a reduced venous distension ensures reduced hepatic bleeding. In addition to this, facilitated mobilization of liver and dissection of hepatic veins are observed. In consideration of findings of improved outcomes, several authors have advocated widespread adoption of the low CVP approach in hepatic resection [13]. Our protocol, aiming at reducing the CVP, includes a limitation of fluid input (4–6 mL/kg/h of balanced crystalloids) along with nitrate infusion (if necessary) to reach the target right atrial pressure. Furthermore, a protective mechanical ventilation strategy (tidal volume of 4–6 mL/kg and positive end-expiratory pressure < 5 cm H2O) contributes to maintaining the CVP in the low range (see Section 3.2).
Although ERAS protocols recommend CVP monitoring for fluid therapy management, we should point out that right atrial pressure (i.e., CVP) represents a marker of how the heart manages the venous return. Therefore, CVP should be taken into account more as a marker of cardiac ability to receive and eject blood forward than as a measurement of volume assessment [20].
Serum lactate (sLac) plays an important role not only because its clearance depends on the liver function, but also because lactatemia is a marker of tissue perfusion and, consequently, an indirect measurement of impaired cellular metabolism [21][22]. During the PM, sLac is expected to rise in a way directly proportional to the single and complete duration of the HH clamping. Hence, it is also intuitive that in case of a low-flow state, a further amount of lactic acid is released into the blood stream due to the cellular metabolism switching into the anaerobic way because of lower oxygen delivery (DO2). Serum lactate (sLac) concentration depends on the balance between production and clearance from the blood stream, and it has been reported to be a predictor of outcome in critically ill patients, including those with liver failure, sepsis and trauma [21][22][23][24][25][26][27][28][29]. Although the peak serum concentration of lactate may correlate with the outcome, its clearance (cLac) seems to be a better predictor [30][31][32].
Hence, we can see how important the continuous hemodynamic assessment is as it aims at avoiding cardiocirculatory imbalance which could affect the postoperative outcome as we found in a retrospective analysis of our database including more than 300 patients forwarded to hepatic resection surgery. In a previous retrospective study, we observed how the intraoperative fluid regimen and the outcome ameliorated after we started monitoring the CO, even when using a semi-invasive method, in the patients forwarded to liver resection [33][34].
2.1. How Much Fluid Does a Patient Need?
The ERAS (Enhanced Recovery After Surgery) protocols recommend that intraoperative fluid load should be limited. At the same time, an excessively restrictive fluid regimen (also in order to limit the intraoperative backflow bleeding) may cause a low-flow state. Modern hemodynamic monitoring based on the heart–lung interaction can predict the response of the patient to fluid administration. It depends on the point of the Frank–Starling curve where the heart of the patient is: at the steep portion of the curve, it will respond positively (i.e., SV increases by 10%); conversely, at the flat portion of the curve, it will not respond. According to this new concept of “functional hemodynamics”, we can predict such a response observing the so called dynamic indices, the most used of which are SVV and PPV (pulse pressure variation, i.e., the percentage of variation of the pulse pressure, that is the difference between systolic and diastolic blood pressure). Since the prediction of fluid responsiveness (FR) obeys the rules of heart–lung interaction caused by the inverted intrathoracic pressure during mechanical ventilation when the surgeon has to open the chest in order to better isolate the liver, the dynamic indices are not reliable. In that case we can observe the stroke volume and the cardiac output to assess hemodynamics [34][35][36][37][38][39]. Last but not least, particularly both during the isolation of the liver and the resection phase, surgical manipulations often cause a stretching of the IVC which reduces the venous blood return and consequently the reduction of the SV and CO. The measurement of the diameter of the IVC by ultrasound performed by the surgeon in the operative field does not help to solve the question of the IVC diameter reliability and helpfulness in fluid management [40].
2.2. How to Manage CVP?
In our opinion, the more the inferior vena cava is “filled”, the less its displacement will cause hemodynamic impairment. The challenge is to find the optimal compromise between fluid overload and an “empty” IVC. The whole integration of the parameters we measure and monitor could address this issue. Our fluid management protocol includes a limitation of fluid input by no more than 4–6 mL/kg/h of balanced crystalloids during the pre-transection and resection phases of surgery. After the resection of the hepatic parenchyma, fluid input is permitted to a limit of 7 mL/kg/h until the patient awakening in the recovery room. Then, the postoperative fluid input consists of 60–80 mL/h as necessary.
Moreover, aiming at giving the appropriate amount of fluids without making the CVP rising (target < 6 mm Hg), nitroglycerine i.v. infusion may be an option. At the same time, since it may provoke hypotension, norepinephrine can guarantee a mean arterial pressure (MAP) > 65 mm Hg and changing the dose rate according to the clamping and unclamping of the hepatic hilum.
Finally, a protective mechanical ventilation strategy (tidal volume of 4–6 mL/kg and positive end-expiratory pressure ≤ 5 cm H2O) avoids excessive rising in intrathoracic pressure and consequently minimizes impact on CVP increase.
3. Intraoperative Acid–Base Balance and Metabolic Issues
The anesthetic management during partial hepatic resection represents a challenge from a metabolic point of view as well. Indeed, the liver is one of the most important organs responsible for detoxifying metabolites, synthesizing proteins and producing biochemical substances involved in homeostasis.
Our goals for liver resection are to avoid the increase of preexisting hepatic disorders and preserve function of the residual liver parenchyma as it may have important effects on the postoperative course in terms of mortality and morbidity [41].
Three aspects need to be considered: (1) patients undergoing liver resections may have different levels of hepatic dysfunction; (2) during parenchymal transection, the surgeon temporarily occludes the inflow to the liver to reduce blood loss (Pringle maneuver) causing hepatic ischemia; (3) liver function changes after resection because of decreased parenchymal volume.
These factors contribute to a variety of metabolic derangements, the most significant of which is lactic acidosis responsible for an increased anion gap and therefore for a progressive worsening of the acid–base balance.
Lactic acid (Lac) is a byproduct of anaerobic metabolism during tissue hypoxia. Normally, Lac is metabolized by the liver (that accounts for up to 50–70% of the whole lactate clearance (cLac)), while the kidneys clear approximately 30–33% and skeletal muscles do the rest. Moreover, serum lactate is an early indicator of impaired tissue microcirculation and it is associated with the outcome as serum Lac > 4 mmol/L persistent after 24 h is associated with a reduced survival in critically ill patients [42]. May a “hepatic-surgery patient” be similar to a critical care patient? We think so.
Chronic liver disease exacerbates hyperlactatemia in general, and this condition could already exist in patients affected by hepatocarcinoma or treated with chemotherapy that induces steatosis. Moreover, during partial hepatectomy with the Pringle maneuver, the liver reduces cLac and becomes itself a lactate producer. The resulting hyperlactatemia determines the occurrence of acidemia that, with concomitant hypoxia, definitely impairs lactate clearance, closing a vicious cycle. Furthermore, hyperlactatemia may be responsible for the reduced cardiac contractility and cardiac output and increased risk of arrhythmias. Intraoperative stresses, blood loss, endogenous release of stress hormones and vasoactive drugs administration may worsen the acid–base balance because these factors increase the amount of pyruvate which is converted into lactate. Serum lactate can also be increased by transfusion of stored blood, which contains some part of lactate, depending on the duration of storage [42].
Assuming appropriate ventilation in intubated patients during hepatic resection, metabolic acidosis can be mild (pH 7.30–7.36), moderate (pH 7.20–7.29) or severe (pH < 7.20). A reduction in extracellular pH alters the opening of proton-gated K+-channels in the myocardium and in blood vessels (along with nitric oxide release), enhancing arrhythmogenicity and contributing to their vasodilatation. Moreover, the opening of pH-gated potassium channels promotes apoptosis directly or acting in combination with hypoxia [41][42].
Normally, the rise in Ca2+ during acidemia contrasts the depressive effects of acidosis on the cardiac function. When we administer bases to counteract acidosis, we reduce ionized calcium levels and it may limit the improvement of the cardiac function despite the amelioration of acidosis. For this reason, it can be worthwhile infusing calcium during the operation [43].
In a previous report, we found a peculiar trend of early postoperative serum lactate concentration. Whatever the PM duration (more or less than 76 min), the postoperative clearance of serum lactate exhibited a particular curve shape that we named the “square root” shape as it draws a line which reminds us of the mathematical sign of the square root (Figure 2) [44].
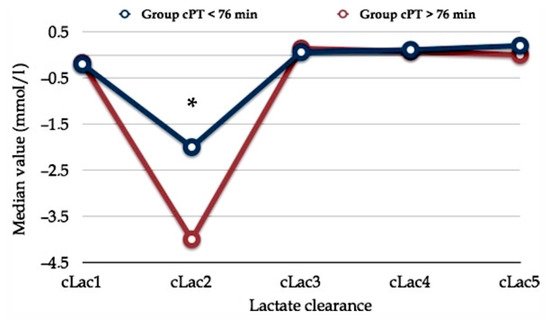
Figure 2. Early postoperative serum lactate clearance. The horizontal axis shows serum lactate clearance (cLac) at different postoperative hours (e.g., cLac1, cLac at the 1st postoperative hour; cLac2, cLac at the 2nd postoperative hour; etc.). The vertical axis shows the relative value of lactate clearance (cLac). Lac clearance was computed using the following formula: (sLac1 – sLac2)/sLac1. Two populations with different Pringle maneuver durations (cumulative Pringle maneuver time, cPT) are plotted. Asterisk (*) means statistical significance, p < 0.05. Comment. Reduced cLac and serum lactate accumulation produce the initial drop into the cLac curve. Serum lactate levels normalize following an increase in effective lactate clearance after the third postoperative hour. These finding persuades us to routinely wait three hours after the awakening of the patient before deciding whether the patient requires postoperative surveillance in the ICU. Abbreviations. cLac, serum lactate clearance; cPT, cumulative Pringle maneuver time. Reprinted with permission from reference [44].
In any case, perioperative management of lactic acidosis, especially when associated with severe acidemia, is an inseparable part of anesthesia, postoperative and critical care management since it presents a variety of challenges. It is of utmost important to maintain adequate organ perfusion, considering that liver capacity for lactate clearance is surely compromised by the ischemic phase due to the Pringle maneuver. This is even more essential if we consider that all general anesthesia techniques in the absence of surgical stimulation reduce the hepatic blood flow by about 30% [41].
In a large multicentric prospective study, Vibert et al. showed the prognostic value of end-surgery serum lactate concentration > 3 mmol/L as an independent predictive risk factor of postoperative complications. Hence, it can help clinicians regarding the need for intensive care unit admission after surgery. In addition, diabetes, repeated hepatectomy, major hepatectomy, major associated procedures besides hepatectomy, inflow occlusion and blood transfusion were the perioperative factors predicting increased lactate concentration [45]. Accordingly, Wiggans et al. concluded that the initial postoperative lactate concentration is a useful predictor of the outcome. In particular, patients with early postoperative serum Lac > 6 mmol/L exhibited a higher 90-day mortality than patients with serum Lac < 2 mmol/L (28% vs. 0.7%, respectively) [46].
To the best of our knowledge, there is no agreement about when, how and how fast lactic acidosis correction should be reached. Given the severity of prognosis linked to a lasting metabolic acidosis, an early cause-oriented treatment is crucial. The goal must be the normalization of blood lactate levels, pursuing the hemodynamic optimization, then tissue perfusion improvement and finally the restoration of proper lactate removal [45].
The use of sodium bicarbonate is simple and inexpensive. It was a cornerstone for acidosis treatment, but its administration in lactic acidosis is not effective and no benefit has been found in terms of clinical outcomes or mortality [47]. The use of sodium bicarbonate in this context is not recommended, but it may be beneficial in cases of severe acidemia in patients with renal failure or complications of advanced liver disease [41][42]. An alternative may be the administration of dichloroacetate. Unfortunately, dichloroacetate is metabolized almost exclusively by the liver in a way involving pyruvate dehydrogenase (PDH). Since in patients with cirrhosis the activity of PDH is reduced, it may be ineffective. Another option may be tromethamine (THAM) as it buffers protons thanks to the ammonia moiety improving the buffering capacity of the blood bicarbonate system. Its dosage is computed using the following Equation (1):
It should be used with caution in patients with impaired renal function (GFR < 30 mL/min) because it would have limited efficacy in proton removal and retention of tromethamine in extracellular fluid may lead to hyperosmolality [41][42].
Finally, a helpful strategy to manage metabolic acidosis during partial hepatic resection consists of increasing the respiratory excretion of CO2. In intubated patients, we can modulate the ventilatory support increasing the respiratory rate or modifying the tidal volume aiming at reducing pCO2.
References
- Wei, A.C.; Greig, P.D.; Grant, D.; Taylor, B.; Langer, B.; Gallinger, S. Survival after hepatic resection for colorectal metastases: A 10-year experience. Ann. Surg. Oncol. 2006, 13, 668–676.
- Belghiti, J.; Hiramatsu, K.; Benoist, S.; Massault, P.; Sauvanet, A.; Farges, O. Seven hundred forty-seven hepatectomies in the 1990s: An update to evaluate the actual risk of liver resection. J. Am. Coll. Surg. 2000, 191, 38–46.
- Jarnagin, W.R.; Gonen, M.; Fong, Y.; DeMatteo, R.P.; Ben-Porat, L.; Little, S.; Corvera, C.; Weber, S.; Blumgart, L.H. Improvement in perioperative outcome after hepatic resection: Analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 2002, 236, 397–406, discussion 406–407.
- Sobol, J.B.; Wunsch, H. Triage of high-risk surgical patients for intensive care. Crit. Care 2011, 15, 217.
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Bøtker, H.E.; Hert, S.D.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431.
- Wijeysundera, D.N.; Pearse, R.M.; Shulman, M.A.; Abbott, T.E.F.; Torres, E.; Ambosta, A.; Croal, B.L.; Granton, J.T.; Thorpe, K.E.; Grocott, M.P.W.; et al. METS study investigators. Assessment of functional capacity before major non-cardiac surgery: An international, prospective cohort study. Lancet 2018, 391, 2631–2640.
- Gawande, A.A.; Kwaan, M.R.; Regenbogen, S.E.; Lipsitz, S.A.; Zinner, M.J. An Apgar score for surgery. J. Am. Coll. Surg. 2007, 204, 201–208.
- Melloul, E.; Hübner, M.; Scott, M.; Snowden, C.; Prentis, J.; Dejong, C.H.; Garden, O.J.; Farges, O.; Kokudo, N.; Vauthey, J.N.; et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2425–2440.
- Hartog, A.; Mills, G. Anesthesia for hepatic resection surgery. Contin. Educ. Anaesth. Crit. Care Pain 2009, 9, 1–5.
- Redai, I.; Emond, J.; Brentjens, T. Anesthetic considerations during liver surgery. Surg. Clin. N. Am. 2004, 84, 401–411.
- Page, A.J.; Kooby, D.A. Perioperative management of hepatic resection. J. Gastrointest. Oncol. 2012, 3, 19–27.
- Genda, T.; Ichida, T.; Sakisaka, S.; Tanaka, E.; Mochida, S.; Ueno, Y.; Inui, A.; Egawa, H.; Umeshita, K.; Furukawa, H.; et al. Assessment Committee of Indication for Transplantation. Survival in patients with Child-Pugh class C cirrhosis: Analysis of the liver transplant registry in Japan. Hepatol. Res. 2017, 47, 1155–1164.
- Torzilli, G.; Donadon, M.; Palmisano, A.; Del Fabbro, D.; Spinelli, A.; Makuuchi, M.; Montorsi, M. Back-flow bleeding control during resection of right-sided liver tumors by means of ultrasound-guided finger compression of the right hepatic vein at its caval confluence. Hepatogastroenterology 2007, 54, 1364–1367.
- Safirstein, R.; Andrade, L.; Vieira, J.M. Acetylcysteine and nephrotoxic effects of radiographic contrast agent—A new use for an old drug. N. Eng. J. Med. 2000, 343, 210–212.
- Ho, K.M.; Morgan, D.J. Meta-analysis of N-acetylcysteine to prevent acute renal failure after major surgery. Am. J. Kidney Dis. 2009, 53, 33–40.
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, T.Y.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early Use of N-acetylcysteine With Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction Reduces Myocardial Infarct Size (The NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903.
- Weisbord, S.D.; Gallagher, M.; Jneid, H.; Garcia, S.; Cass, A.; Thwin, S.S.; Conner, T.A.; Chertow, G.M.; Bhatt, D.L.; Shunk, K.; et al. PRESERVE Trial Group. Outcomes After Angiography With Sodium Bicarbonate and Acetylcysteine. N. Eng. J. Med. 2018, 378, 603–614.
- Lentschener, C.; Ozier, Y. Anaesthesia for elective liver resection: Some points should be revisited. Eur. J. Anaesthesiol. 2002, 19, 780–788.
- Hofer, C.K.; Cannesson, M. Monitoring Fluid Responsiveness. Acta. Anesthesiol. Taiwanica 2011, 49, 59–65.
- Magder, S. Volume and its relationship to Cardiac Output and Venous Return. Crit. Care 2016, 20, 271.
- Levy, B. Lactate and shock state: The methabolic view. Curr. Opin. Crit. Care 2006, 12, 315–321.
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Eng. J. Med. 2014, 371, 2309–2319.
- Chioléro, R.; Tappy, L.; Gillet, M.; Revelly, J.P.; Roth, H.; Cayeux, C.; Schneiter, P.; Leverve, X. Effect of major hepatectomy on glucose and lactate metabolism. Ann. Surg. 1999, 229, 505–513.
- Husain, F.A.; Martin, M.J.; Mullenix, P.S.; Steele, S.R.; Elliott, D.C. Serum lactate and base deficit as predictors of mortality and morbidity. Am. J. Surg. 2003, 185, 485–491.
- Khosravani, H.; Shahpori, R.; Stelfox, H.T.; Kirkpatrick, A.W.; Laupland, K.B. Occurrence and adverse effect on outcome of hyper- lactatemia in the critically ill. Crit. Care. 2009, 13, R90.
- Macquillan, G.C.; Seyam, M.S.; Nightingale, P.; Neuberger, J.M.; Murphy, N. Blood lactate but not serum phosphate levels can predict patient outcome in fulminant hepatic failure. Liver Transpl. 2005, 11, 1073–1079.
- Bernardin, G.; Pradier, C.; Tiger, F.; Deloffre, P.; Mattei, M. Blood pressure and arterial lactate level are early indicators of short- term survival in human septic shock. Intensive Care Med. 1996, 22, 17–25.
- Manikis, P.; Jankowski, S.; Zhang, H.; Kahn, R.J.; Vincent, J.L. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am. J. Emerg. Med. 1995, 13, 619–622.
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528.
- Trzeciak, S.; Dellinger, R.P.; Chansky, M.E.; Arnold, R.C.; Schorr, C.; Milcarek, B.; Hollenberg, S.M.; Parrillo, J.E. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007, 33, 970–977.
- Jones, A.C.; Shapiro, N.I.; Trzeciak, S.; Arnold, R.C.; Claremont, H.A.; Kline, J.A.; for the Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA 2010, 303, 739–746.
- Ghneim, M.H.; Regner, J.L.; Jupiter, D.C.; Kang, F.; Bonner, G.L.; Bready, M.S.; Frazee, R.; Ciceri, D.; Davis, M.L. Goal directed fluid resuscitation decreases time for lactate clearance and facilitates early fascial closure in damage control surgery. Am. J. Surg. 2013, 206, 995–999.
- Giustiniano, E.; Procopio, F.; Ruggieri, N.; Grimaldi, S.; Torzilli, G.; Raimondi, F. Impact of the FloTrac/VigileoTM monitoring on intraoperative fluid management and outcome after Liver resection. Dig. Surg. 2018, 35, 435–441.
- Benes, J.; Chytra, I.; Altmann, P.; Hluchy, M.; Kasal, E.; Svitak, R.; Pradl, R.; Stepan, M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: Results of prospective randomized study. Crit. Care 2010, 14, R118.
- Gorcott, M.P.W.; Mythen, M.G.; Gan, T.J. Perioperative fluid management and clinical outcome in adult. Anesth. Analg. 2005, 100, 1093–1106.
- NHS-Technology Adoption Centre (NTAC). Intraoperative Fluid Management Technologies (IOFMT). 2012. Available online: (accessed on 9 February 2021).
- Pearse, R.M.; Harrison, D.A.; MacDonald, N.; Gillies, M.A.; Blunt, M.; Ackland, G.; Grocott, M.P.; Ahern, A.; Griggs, K.; Scott, R.; et al. OPTIMISE Study Group. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA 2014, 311, 2181–2190.
- Brandstrup, B.; Tønnesen, H.; Beier-Holgersen, R.; Hjortsø, E.; Ørding, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648.
- Pinsky, M.R.; Peyen, D. Functional Hemody-namic Monitoring. In Update in Intensive Care Medicine; Vincent, J.L., Ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2011.
- Giustiniano, E.; Procopio, F.; Morenghi, E.; Rocchi, L.; Fabbro, D.D.; Ruggieri, N.; Zito, P.C.; Donadon, M.; Torzilli, G.; Raimondi, F. Does Inferior-Vena-Cava Collapsibility Correlate with Fluid Regimen and Outcome in Patients Undergoing Liver Resection? J. Anesth. Clin. Res. 2015, 6, 577.
- Wilks, J.A.; Hancher-Hodges, S.; Gottumukkala, V.N.R. Contemporary Perioperative Anesthetic Management of Hepatic Resection. Adv. Anesth. 2016, 34, 85–103.
- Vitin, A.A.; Azamfirei, L.; Tomescu, D.; Lang, J.D. Perioperative Management of Lactic Acidosis in End-Stage Liver Disease Patient. J. Crit. Care Med. 2017, 3, 55–62.
- Kraut, J.A.; Madias, N.E. Treatment of acute metabolic acidosis: A pathophysiologic approach. Nat. Rev. Nephrol. 2012, 8, 589–601.
- Giustiniano, E.; Procopio, F.; Costa, G.; Rocchi, L.; Ruggieri, N.; Cantoni, S.; Zito, P.C.; Gollo, Y.; Torzilli, G.; Raimondi, F. Serum lactate in liver resection with intermittent Pringle maneuver: The “square-root” shape. J. Hepatobiliary Pancreat. Sci. 2017, 24, 627–636.
- Vibert, E.; Boleslawski, E.; Cosse, C.; Adam, R.; Castaing, D.; Cherqui, D.; Naili, S.; Regimbeau, J.M.; Sa Cunha, A.; Truant, S.; et al. Arterial Lactate Concentration at the End of an Elective Hepatectomy Is an Early Predictor of the Postoperative Course and a Potential Surrogate of Intraoperative Events. Ann. Surg. 2015, 262, 787–792.
- Wiggans, M.G.; Starkie, T.; Shahtahmassebi, G.; Woolley, T.; Birt, D.; Erasmus, P.; Anderson, I.; Bowles, M.J.; Aroor, S.; Stell, D.A. Serum arterial lactate concentration predicts mortality and organ dysfunction following liver resection. Perioper. Med. 2013, 2, 21.
- Kim, H.J.; Son, Y.K.; An, W.S. Effect of Sodium Bicarbonate Administration on Mortality in Patients with Lactic Acidosis: A Retrospective Analysis. PLoS ONE 2013, 8, e65283.





