Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Chin Hua Chia.
Plants have been used for multiple purposes over thousands of years in various applications such as traditional Chinese medicine and Ayurveda. The special properties of phytochemicals within plant extracts have spurred researchers to pursue interdisciplinary studies uniting nanotechnology and biotechnology. Plant-mediated green synthesis of nanomaterials utilises the phytochemicals in plant extracts to produce nanomaterials. Principles of plant-mediated Cu-based nanomaterials in biomedical and environmental applications are discussed.
- Cu-NMs
- plant extract
- antibacterial activity
1. Introduction
Cu-NMs synthesised using plant extracts have been utilised in two major areas, namely, biomedical and environmental remediation [31][1].
2. Biomedical
Plant-mediated Cu-NMs have demonstrated antimicrobial, antioxidant, and anticancer activities, and have potential as nano-sensors and in various medical applications. In this section, details and mechanisms pertaining to this area will be discussed.
2.1.1. Antimicrobial
2.1. Antimicrobial
Firstly, antibacterial activity has been observed for plant-mediated Cu-NMs [205][2] and can be attributed to several putative pathways. Bhavyasree and Xavier suggested that Cu-NMs, including both Cu and CuO nanoparticles produced via plant-mediated synthesis, can carry out antibacterial activity through a chemisorption-based mechanism [206][3]. This mechanism involves microbial adsorption to the nanoparticle surface, which has been bio-functionalised by phytochemicals during the plant-mediated synthesis process. The adsorption is mainly due to chemisorption via non-electrostatic forces (Van der Waals force and hydrogen bonding), which causes the destruction of the microbial cell wall and subsequent cell membrane damage, DNA breakage, and eventually cell death, as illustrated in Figure 51.
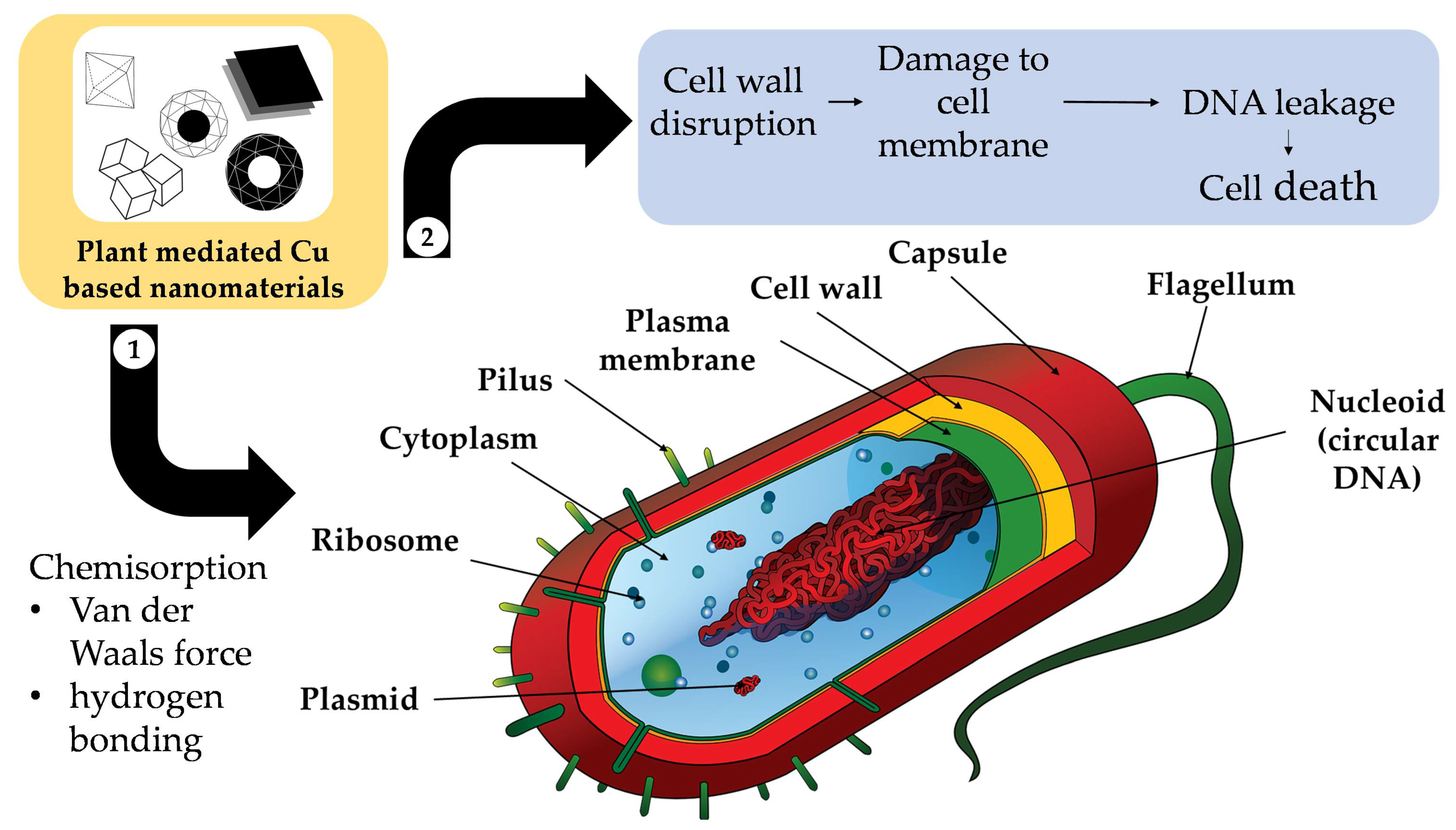
Figure 51.
Diagram of the chemisorption-based mechanism of Cu-based nanomaterials’ antimicrobial activity.
Another antibacterial mechanism is mediated by reactive oxygen species (ROS) and the release of Cu2+ ions [207][4]. First, the CuO nanoparticles are much smaller (being of a nanometre scale) than the micrometre-scale pores of bacterial cells, which allows them to easily penetrate the cells. In addition, Cu2+ ions are attracted toward bacterial cells due to the abundance of carboxyl and amine groups on the cell surface; this is another factor in antibacterial ability. However, the antibacterial interactions are different for Gram-positive and Gram-negative bacteria, as described in Figure 62a.
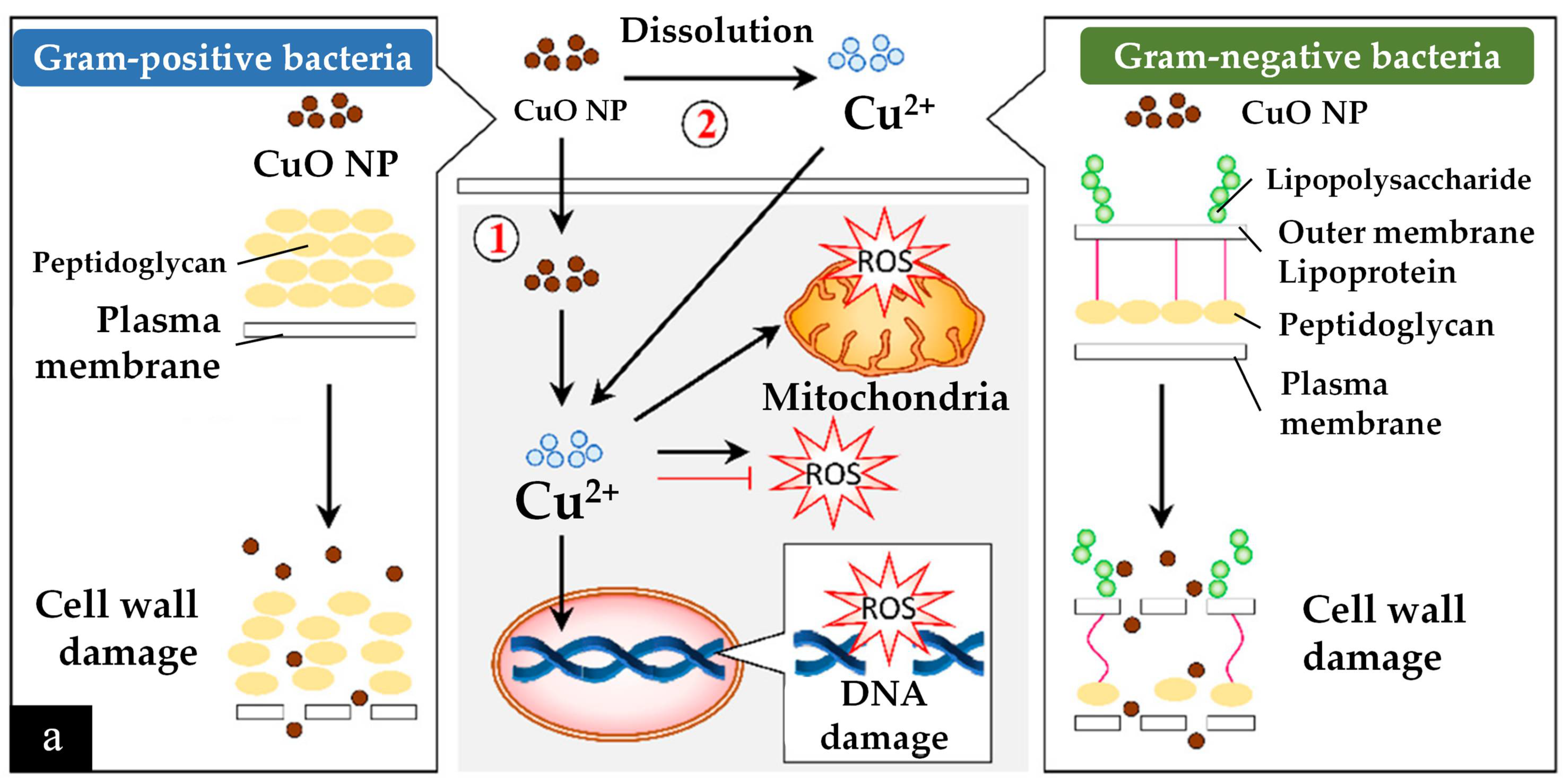

After bypassing the cell wall, Cu2+ ions relocate intracellularly to the cytosol due to the internalization of CuO nanoparticles and Cu2+ ions, where they cause ROS to accumulate [205][2]. Consequently, DNA and mitochondria damage occur. Cu2+ ions within a bacterium may also stimulate cellular responses that lead to bactericidal activity. For example, radicals produced by CuO nanoparticles, such as superoxide and hydroxyl radicals, can have synergic effects in causing bacterial membrane destruction, DNA damage, attachment to ribosomes, oxidative injury, and protein and proton efflux pump damage; they can also prevent biofilm production [205,207][2][4].
Cu-NMs exert antibacterial activity through mechanisms similar to those of Cu and CuO nanoparticles. Generally, the antibacterial activity of nanomaterials is mainly owed to the induction of oxidative stress, such as through the production of free radicals and ROS. Notably, nano-sized particles will feature a smaller surface-to-volume ratio, harbour more surface defects due to oxygen vacancies, and feature greater electrostatic attraction and release of Cu2+ ions and generate more oxidative stress within the bacterial cells [178][5]. In addition, bimetallic nanoparticles can demonstrate a synergic effect with improved antibacterial ability. For example, bimetallic Ag and Cu nanoparticles produced by Vitex negundo-mediated synthesis demonstrate antibacterial activity when applied in a cellulose matrix via the disc method against both Gram-positive (Escherichia coli, Pseudomonas, Klebsiella) and Gram-negative (Staphylococcus, Bacillus) species [178][5]. Particles having an equal ratio of Ag and Cu (2.5 mM each) exhibited the greatest antibacterial ability, with a 9 mm zone of inhibition for all the tested species.
Secondly, Cu-NMs have also been demonstrated to possess antifungal activity. A number of fungi can cause infections in humans with severe symptoms, such as Candida albicans which can cause mucosal infections (oropharyngeal or vulvovaginal candidiasis), or Trichophyton mentagrophytes which can cause dermatophytosis [208,209][6][7]. Antifungal activity is more challenging to realise than antibacterial as a fungus cell has several layers of lipids within its cell wall which impede the penetration and internalization of Cu nanomaterials [205][2]. Although fewer publications exist regarding the antifungal testing of Cu nanomaterials synthesised by green methods, their hypothesised antifungal mechanism is based on altering the structure and function of fungal cell components [210][8]. That is, the nanoparticles first distort the cell wall and become internalised by the fungus (Figure 62b). After internalization, the same process of ROS generation and subsequent process disruption ensues as in bacteria, impacting DNA, mitochondria, replication, protein synthesis and other essential elements, eventually leading to cell death [205,210][2][8].
In one report of Cu-NM antifungal activity, Mali et al. [210][8] tested the efficacy of Cu nanoparticles derived from Celastrus paniculatus leaf extract against Fusarium oxysporum. Concentrations of 0.12%, 0.18% and 0.24% (w/v) Cu nanoparticles were found able to inhibit mycelial growth by 76.29 ± 1.52%, 73.70 ± 1.52%, and 59.25 ± 0.57%, respectively, calculated via the following formula:
(% inhibition rate)=(Mc−Mt)Mc×100
Dobrucka and Dlugaszewska similarly studied the antibacterial and antifungal activities of Cu-Pt core-shell nanoparticles synthesised using Agrimoniae herba extract [170][9]. The nanoparticles were applied via the well-diffusion method to three species of bacteria, including Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, and three of fungi: Candida albicans, Trichophyton mentagrophytes, and Aspergillus fumigatus; the authors then determined the minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC), and minimal fungicidal concentration (MFC) [170][9]. The Cu-Pt nanoparticles exhibited good inhibitory function on all tested bacteria and Trichophyton Mentagrophytes. The overall best antibacterial and antifungal performances were obtained on Staphylococcus aureus (MIC of 16.7 and MBC of 33.3) and Trichophyton mentagrophytes (MIC and MFC of 26.7).
From the above reports, it can be concluded that plant-mediated Cu-NMs are suitable as antibacterial (for both Gram-positive and -negative) and antifungal agents. Such characteristics are useful in further broadening the application of Cu-NMs in the pharmaceutical and medical sectors.
In addition to direct antimicrobial effects, many plant-mediated Cu-NMs have also demonstrated antioxidant properties which also contribute to antibacterial and antifungal activities as a synergic factor [205][2]. Multiple mechanisms contribute to antioxidant ability, which are: (1) binding of transition metal ion catalysts, (2) reductive capacity, (3) radical scavenging activity, (4) decomposition of peroxides, (5) prevention of continued hydrogen abstraction, and (6) prevention of chain initiation.
Interestingly, plant selection has been shown to impact the antioxidant ability of Cu-NMs. For example, Rehana et al. [211][10] synthesised nanoparticles using extracts of Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, and Tamarindus indica, then tested their antioxidant capabilities with ABTS, DPPH, and hydrogen peroxide assays. Tamarindus indica-mediated nanoparticles were found to have the highest antioxidant activity, and Moringa oleifera the lowest, though still superior to CuO nanoparticles produced via a chemical method. Therefore, plant-mediated nanomaterials have much higher antioxidant ability as compared to chemical-mediated materials, and the plant used is an essential consideration for antioxidant purposes.
2.1.2. Nano-Sensor
2.2. Nano-Sensor
Plant-extract-mediated Cu-NMs have also been utilised in the preparation of nano-sensors. Cu nanomaterials, such as CuO nanoparticles, are suitable for nano-sensor production owing to their characteristic high electron-transfer rate, superior catalytic activity, large surface area, high glucose selectivity in heterogenous samples (such as blood or urine), chlorine poisoning resistance, and corrosion resistance. For example, Ag-CuO core-shell nanoparticles produced using Ocimum tenuiflorum extract have been used for non-enzymatic glucose sensing with a screen-printed electrode [174][11]. The synthesised electrode provided good glucose-sensing performance with a sensitivity of 3763.44 µAmM−1cm−2, linear range of 1 to 9.2 mM, detection limit of 0.006 mM (S/N = 3), and response time of less than 1 s. Moreover, the CuO-Ag core-shell-modified bio-nano-sensors demonstrated exceptional adhesion and structural strength along with great long-term stability for up to 60 days, exhibiting 99.2% of the initial value after one month with excellent repeatability and reproducibility.
The mechanism by which these nanoparticles sense glucose is based on electron transfer from the screen-printed electrode to the CuO nanoparticle core via the conduction band electrons of the Ag shell. This electron transfer occurs because the work function of CuO is bigger than that of Ag, and equalization of Fermi levels ensues after the materials come into electrical contact and the mobility of electrons is improved. The progression of current-induced charge carriers can boost electrocatalytic efficiency through a charge transfer mechanism; therefore, the Ag-CuO core-shell nanoparticles are electro-catalytically active and can induce electron-transfer reactions. The energy of a nanoparticle is dependent on the charge distribution within the energy levels of its component metal. Ultimately, the additional electrons can be discharged when glucose is introduced into the system as an electron acceptor.
This glucose oxidation mechanism can be summarised as: (1) deprotonation of glucose that causes oxidation, (2) isomerization and enediol formation, and, finally, (3) adsorption to the electrode surface, which leads to the oxidation of Cu(II)/Cu(III):
CuO+OH− → CuOOH+e−
CuOOH+glucose+ e−→ CuO+OH−+gluconic acid
In the core-shell nanoparticle, Cu(II) was oxidised to Cu(III) and this catalysed glucose oxidation to produce gluconolactone, which was further oxidised to gluconic acid as presented in Equations (2) and (3).
2.1.3. Anticancer
2.3. Anticancer
Lastly, plant extract-mediated Cu-NMs have been studied for their anticancer properties. Generally, this activity can be realised through multiple routes including ROS generation, antioxidant activity, cell cycle arrest, apoptosis, and autophagy [205,207][2][4]. One study produced CuO nanoparticles using extracts of Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, and Tamarindus indica and used MTT assays to test their activity against four cancer cell lines, i.e., human breast, cervical, epithelioma, and lung cancer cells, along with one normal human dermal fibroblast (NHDF) cell line [211][10]. All CuO nanoparticles exhibited anticancer ability towards all cancer cell types in a dose-dependent manner: higher concentrations of CuO nanoparticles resulted in lower cancer cell viability. Interestingly, the type of plant utilised also affected anti-cancer ability, with Tamarindus indica-mediated CuO nanoparticles exhibiting greater cytotoxicity over the others; this indicates that the phytochemicals in the plant extract used for nanoparticle synthesis impact the resulting particles’ anti-cancer activity.
The toxicity of Cu-NMs is one of the limitations that hinder their application biomedically. However, it has been reported that plant-mediated Cu nanomaterials have less toxicity to normal human cell lines [205][2]. Therefore, such Cu nanomaterials may be more safely applied in biomedical applications. For example, CuO nanoparticles synthesised using extracts of Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, and Tamarindus indica exhibited lower toxicity in NHDF cells, which suggests these to be promising anticancer agents for use in the pharmaceutical industry [211][10].
2.2. Environmental Remediation
3. Environmental Remediation
The usage of Cu-NMs in environmental applications is mainly focused on the remediation of dyes and toxic compounds, with mechanisms primarily based on photocatalysis or catalysis.
The mechanism of photocatalysis by nanomaterials is as follows: when the nanomaterials are deposited into an aqueous sample containing compounds that are desired to be degraded, such as dye, and exposed to light, an interaction occurs in which a photogenerated electron is converted from the valence band (VB) to the conduction band (CB) in the nanomaterial. A hole in the VB then results, producing an electron (e−)-hole (h+) pair. The holes react with OH ions in the water molecules to yield OH radicals via oxidation, while the electrons react with dissolved O2 to generate O2 radicals via reduction. Those radicals are then responsible for the degradation of the dye into non-toxic degraded products [171,176][12][13]. Alshehri and Malik investigated the ability of Origanum vulgare extract-mediated Cu-Co-Ni trimetallic nanoparticles to photocatalyse the degradation of methylene blue [176][13]. They observed degradation efficiency of more than 50% and 92.67% after 50 and 100 min, respectively. The rate of degradation could be increased via increasing nanoparticle concentration, but after a certain threshold was surpassed, the photocatalytic efficiency could be enhanced no further due to the aggregation of the nanomaterials.
With regard to catalysis mechanisms, nanoparticles can catalyse reactions by borylation, clock reactions, oxidative coupling, A3 coupling, click chemistry, tandem and multicomponent reactions, C–H functionalization, cross-coupling, reduction and oxidation reactions, and other mixed reactions [212][14]. Successful catalysis via plant-mediated Cu-NMs has been achieved, such as when Suvarna et al. [173][15] studied the degradation of methyl green dye using bimetallic spherical Fe-Cu nanoparticles produced using Cyclea peltata extract, and achieved a degradation efficacy of 82% within 105 min. The Fe-Cu nanoparticles promoted hydrolysis and deprotonation reactions on the dye molecules, resulting in the demineralization of the dye molecules into simpler structures. In another example, Rosbero and Camacho utilised bimetallic (Ag and Cu) alloy nanoparticles produced via Carica papaya leaf extract to degrade the pesticide chlorpyrifos in water [172][16]. The degradation was observed for 24 h, and yielded the products 3,5,6-trichloropyridinol (TCP) and diethylthiophosphate (DETP), of which the former is less toxic than chlorpyrifos and not mutagenic.
References
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577.
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564.
- Bhavyasree, P.G.; Xavier, T.S. Green Synthesised Copper and Copper Oxide Based Nanomaterials Using Plant Extracts and Their Application in Antimicrobial Activity: Review. Curr. Res. Green Sustain. Chem. 2022, 5, 100249.
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508.
- Mamatha, G.; Sowmya, P.; Madhuri, D.; Mohan Babu, N.; Suresh Kumar, D.; Vijaya Charan, G.; Varaprasad, K.; Madhukar, K. Antimicrobial Cellulose Nanocomposite Films with In Situ Generations of Bimetallic (Ag and Cu) Nanoparticles Using Vitex negundo Leaves Extract. J. Inorg. Organomet. Polym. Mater. 2021, 31, 802–815.
- Klinger, M.; Theiler, M.; Bosshard, P.P. Epidemiological and Clinical Aspects of Trichophyton Mentagrophytes/Trichophyton Interdigitale Infections in the Zurich Area: A Retrospective Study Using Genotyping. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1017–1025.
- D’Enfert, C.; Kaune, A.-K.; Alaban, L.-R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The Impact of the Fungus-Host-Microbiota Interplay upon Candida albicans Infections: Current Knowledge and New Perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060.
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green Synthesis of Copper Nanoparticles Using Celastrus paniculatus Willd. Leaf Extract and Their Photocatalytic and Antifungal Properties. Biotechnol. Rep. 2020, 27, e00518.
- Dobrucka, R.; Dlugaszewska, J. Antimicrobial Activity of the Biogenically Synthesized Core-Shell Nanoparticles. Saudi Pharm. J. 2018, 26, 643–650.
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of Antioxidant and Anticancer Activity of Copper Oxide Nanoparticles Synthesized Using Medicinally Important Plant Extracts. Biomed. Pharmacother. 2017, 89, 1067–1077.
- Dayakar, T.; Venkateswara Rao, K.; Park, J.; Krishna, P.; Swaroopa, P.; Ji, Y. Biosynthesis of Core–Shell Nanostructures for Non-Enzymatic Glucose Sensing Using Screen-Printed Electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 9725–9734.
- Rajendaran, K.; Muthuramalingam, R.; Ayyadurai, S. Green Synthesis of Ag-Mo/CuO Nanoparticles Using Azadirachta indica Leaf Extracts to Study Its Solar Photocatalytic and Antimicrobial Activities. Mater. Sci. Semicond. Process. 2019, 91, 230–238.
- Alshehri, A.A.; Malik, M.A. Facile One-Pot Biogenic Synthesis of Cu-Co-Ni Trimetallic Nanoparticles for Enhanced Photocatalytic Dye Degradation. Catalysts 2020, 10, 1138.
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811.
- Suvarna, A.R.; Shetty, A.; Anchan, S.; Kabeer, N.; Nayak, S. Cyclea Peltata Leaf Mediated Green Synthesized Bimetallic Nanoparticles Exhibits Methyl Green Dye Degradation Capability. Bionanoscience 2020, 10, 606–617.
- Rosbero, T.M.S.; Camacho, D.H. Green Preparation and Characterization of Tentacle-like Silver/Copper Nanoparticles for Catalytic Degradation of Toxic Chlorpyrifos in Water. J. Environ. Chem. Eng. 2017, 5, 2524–2532.
More
