Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mohd Hazim Mohamad Amini and Version 2 by Catherine Yang.
Wood is an excellent building material or component that has been used all over the world. The rise in energy consumption worldwide, particularly in the building sector, has led to the development of diverse methods to overcome this problem. Embedding phase change material, phase change material (PCM), into the wood has been researched as one of the most effective alternatives of controlling the thermal loads of wood, as it can store and release latent heat energy at a specific temperature range.
- wood
- phase change material
- latent heat
1. Phase Change Material (PCM)
Phase change material (PCM) is described as a material that is able to store and release heat energy (known as latent heat energy) through its physical phase change from solid to liquid and vice versa [1][21]. The heat energy of a substance is considered latent because it is stored between molecules until it changes from one phase to another. The matter comprises molecules, which are held together by chemical bonds. Those chemical bonds are used as heat stores and releases. Physical changes in matter can occur when the matter goes through phase changes. The phase changes of material happen due to the changes in its temperature when it is heated or cooled within its specific temperature range. A PCM works by storing (absorbing) heat energy as it is heated, breaking down the bonding responsible for the solid structure and changing it to a liquid state [2][22].
Meanwhile, energy is released as the PCM cools down and changes from liquid to solid. A PCM’s functioning process is illustrated as in Figure 12. The action of releasing and absorbing energy happens only with latent heat energy, without changing the temperature of the PCM.
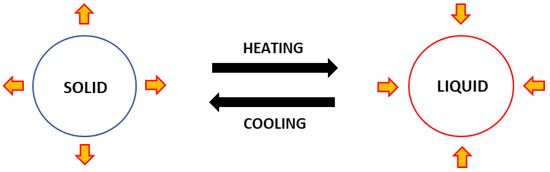
Figure 12.
Illustration concept of phase change material.
Latent heat is the energy required to change a given substance from one physical state to another at a given temperature [3][23]. Because of the tremendous amount of energy needed to move from one physical state to another, PCM is considered an efficient source of latent heat energy storage. Therefore, PCM is gaining attention regarding its application in the building sector as an effective way to improve the building envelopes, which can resolve the contradiction between the energy demand and supply efficiently and reduce energy consumption effectively. Generally, all materials are phase change materials since they experience phase transformation at a certain temperature range. Yet not all materials are fit for storing latent heat energy. Several characteristics are required for a material to be used in thermal energy storage. High thermal conductivity, high latent heat of melting, low vapour pressure, high density, chemical inertia and stability, nontoxicity, non-flammability, non-corrosivity, cost efficiency, congruent melting and cooling, minimal subcooling, and a phase transition that occurs in the practical range of operation are the requirements for the material to be used as a thermal regulator component [4][24]. In selecting a PCM for any application, the heating and cooling operating temperature should match the PCM’s phase transition temperature. Most of the PCMs used do not satisfy the required criteria for thermal energy storage. Meanwhile, PCM criteria, such as melting temperature, cost, toxicity, flammability, and stability, were taken into account in the building sector. The main disadvantages of pure PCM are leakage and low thermal conductivity, which limits their practical energy storage efficiency [5][25]. Materials such as wood powder and porous cellulose nanofibril hybrid supporting materials could increase their thermal conductivity [6][26].
2. Classification of Phase Change Materials
PCMs are categorized into three classes, which are organic, non-organic, and eutectic [7][8][27,28]. A detailed classification of PCM is depicted in Figure 23.
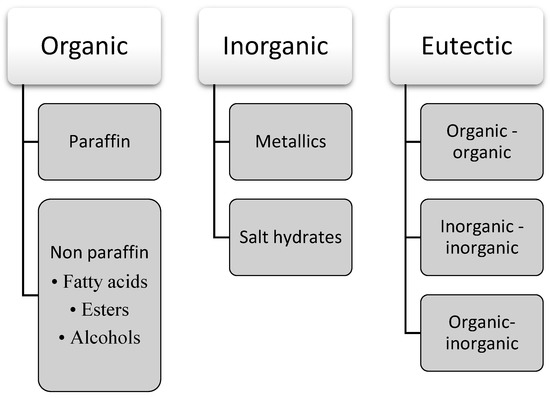
Figure 23.
Detailed classification of PCM.
The organic PCM mainly consists of paraffin, and non-paraffin material comprises fatty acids, esters, and alcohols. Natural paraffin primarily contains a mixture of pure alkanes with a wide range of phase change temperatures. Of these, paraffin wax is an organic PCM mainly used commercially. Paraffin wax is insoluble in water and does not react with most common chemical reagents. Meanwhile, researchers use non-paraffin as the most favourable PCM due to its availability in an extensive range of temperatures and a high fusion heat. Fatty acids are the most common inorganic PCM used. The confirmation of its continuous supply due to its sources that come from common vegetables and animal oil is one of the factors that make fatty acids the most favourable PCM under the non-paraffin group.
Salt hydrate and metallic are included in the inorganic PCM group. Metal is the most minor preferred type of PCM due to its low latent heat and weight penalties. However, metal still can give some advantages, especially in applications where compactness is the main goal due to its high heat of fusion per unit volume. Aluminium, zinc, magnesium, and their alloys are fit to be used in an application with high temperatures. Meanwhile, indium, cesium, bismuth, gallium, and tin are types of metals that can be used in applications with low temperatures. Additionally, metal has high thermal conductivity as well as high physical and chemical stability. Salt hydrates are an ionic compound with a three-dimensional structure. The salt hydrate ions can attract several water molecules and enclose them inside their crystal lattice. Despite several advantages offered by salt hydrates over the organic PCM, the chemical instability of salt hydrates limits its broad utilization. They possess a high tendency of phase separation due to their primary components comprising salt and water. Water is lost after every heating cycle, while hydrated salts tend to settle out during the melting process. However, this issue can be fixed to a certain extent using thickened or gelled mixtures. The other significant drawback of salt hydrates is supercooling. A high degree of supercooling prevents salt hydrates from crystallizing at the specified freezing point. Using suitable nucleating agents that can trigger the crystal growth in the storage medium can help with supercooling issues.
Lastly, the mixture of two or more organic, inorganic, or both compounds are classified as a eutectic group. The advantage carried out by eutectic PCM is the preferable selective combination of excellent performances between these two compounds. They have a higher density than organic PCM and form a crystal during crystallization. Eutectic mixtures freeze and melt as a single compound to an intimate mixture of crystals simultaneously, without undergoing phase separation. Countless eutectics can be produced for any preferred melting point. It can be seen that most eutectics for PCMs are developed from the mixture of salt hydrates and fatty acids, respectively. However, recent research has turned to producing eutectics from organic groups due to salt hydrates’ phase separation and supercooling issues [9][29]. Still, eutectic PCM’s thermal and physical properties data are still inadequate due to their recent introduction. The advantages and disadvantages of the organic, inorganic, and eutectic groups are displayed in Table 1.
| Organics | Inorganics | Eutectics | |
|---|---|---|---|
| Advantages | High heat of fusion | High heat of fusion | Sharp melting temperature |
| Available in large temperature range | High thermal conductivity | High volumetric thermal storage density | |
| Minimal supercooling (self-nucleation) | Low vapor pressure in the melt state | ||
| Melt and freeze repeatedly without phase segregation | Sharp melting point | ||
| Congruent phase transition process | High volumetric latent heat storage capacity | ||
| High thermal stability | Low volume change | ||
| Non-corrosive, non-reactive | Non-corrosive, nonreactive and non-flammable | ||
| Recyclable | Low cost and easy availability | ||
| Chemically stable | Good compatibility with conventional construction materials | ||
| Drawbacks | Expensive | Slightly toxic in nature | Highly expensive |
| Low thermal conductivity | Supercooling (low degree of nucleation) | ||
| Low density | Corrosive with some metals | ||
| Least compatible with plastic containments | Dehydration occurs during the phase change process | ||
| Flammable (depending on the containment) | Compatibility with some building materials is limited | ||
| Low volumetric latent heat storage capacity | High volume change | ||
| Examples | Paraffin wax (Paraffin type) [12][13][32,33] | Fields’ metal (32.5Bi/51In/16.5Sn wt%) and 49Bi/18Pb/12Sn/21In wt% (Metallic type) [14][34] | Mixture of lauric and stearic acid [15][35] |
| Capric acid (Non-paraffin, Fatty acid type) [16][36] | Sodium sulfate decahydrate (SSD) salt hydrate (Salt hydrates) [17][37] | Oleic-Myristic acid eutectic PCM [18][38] | |
| Stearic acid (Non-paraffin, Fatty acid type) [19][39] | Na2HPO4·12H2O hydrated salt (Salt hydrates) [20][40] | Methyl palmitate and lauric acid eutectic mixture [21][41] | |
| Palmitic acid (Non-paraffin, Fatty acid type) [22][42] | NaNO3/KNO3 (Salt hydrates) [23][43] | Lauric acid/myristyl alcohol eutectic mixture [24][44] | |
| Hydrogenated palm stearin (Non-paraffin, Ester type) [25][45] | Palmitic acid-stearic acid/CuO nanoparticles [26][46] | ||
| Lauryl alcohol (Non-paraffin, alcohol type) [27][47] |
3. Impregnation and Evaluation of Phase Change Material in Wood
Leaking and low thermal conductivity are common issues encountered as PCM is used directly in the application. Thus, a porous shape stabilizer or supporting materials are required to hold the PCM so that PCM can serve as energy storage efficiently. Porous supporting materials can adsorb and keep the PCM through several mechanisms, including capillary force, surface tension, and other connections between PCM and supporting materials. However, the inherent nanoconfinement effect of supporting materials and the melting and freezing enthalpies of the composite PCMs are usually lower than that of bulk PCMs.
Another drawback of pure PCM is its inherent low thermal conductivity, resulting in a hysteretic thermal response. Assembling PCM with thermally conductive materials is a common way to improve thermal conductivity. Metallic substances, metal foams, carbon nanotubes, and graphene are some of the materials combined with the PCM to make a more thermally conductive composite. However, the thermally conductive additive also tends to fall off the composite during the thermal cycle due to changes in interfacial contact. Moreover, the thermal conductivity additives and energy storage density have an opposite relationship, where increasing the additive content will lower the energy storage density [28][48]. The problem of producing the composite in large scale is also not cost effective, thus limiting its application.
Biomass is a promising material as a replacement for the mentioned materials. Biomass, mainly wood, is a renewable, abundant, and highly cost-effective resource. Wood has a pore structure ranging from millimetres to nanometers, which can be used as supporting material for PCM. The hydrogen bonding between the porous structure and PCM provides good interaction to prevent the PCM from leaching out during thermal cycles. The wood can be carbonized to increase its pore structure further, increasing its thermal conductivity. Shape-stabilized PCM using carbonized biomass has recently been extensively researched [29][30][49,50].
Besides carbonization, delignification can also improve the PCM holding capacity of the wood. Delignified wood has a bigger pore size and porosity, which makes the impregnation process easier. Whiter bleached wood also can be fabricated into another functional wood composite. Transparent and thermal regulative wood composite has been manufactured using epoxy resin, polyethylene glycol, and delignified wood [31][51]. Another transparent and thermal regulative wood composite with thermochromic properties has been fabricated by Yang et al. [32][52]. The composite showed good enthalpy at about 100 J/g and survived 100 heating and cooling cycles without any leakage. It proves that wood exhibits good thermal energy management ability. The increment in PCM immersion depth also increases the interface resistance between phase change materials and wood cells, as been researched by [33][53]. Good mechanical properties, excellent heat management ability, and suitable phase change temperature are some characteristics of wood-stabilized PCM for outdoor building energy conservation and management [34][54].
The unique characteristics of wood have brought back an interest in utilizing wood as construction materials during the last decades. To achieve the primary goal of reducing energy consumption, the application of great thermal energy storage systems, PCM particularly, into the wood, is still growing in interest. The anisotropic microstructure and porous structure with various pore diameters (micropores, mesopores, and macropores) make wood a suitable shape stabilizer or supporting material for PCMs. The micropores can induce the adsorption of PCM through physical forces, such as surface tension and capillary forces. The transport channel mechanism happens inside the mesopores and macropores, especially for the melted PCM [5][25]. Virgin wood possesses a natural pipe and ordered pore structure that is better for mass and energy transmission, especially in an environment that requires anisotropic heat conduction. They have good compatibility with PCM, inherent low thermal conductivity and can serve as better shape stability and security to the PCM. The other advantage of using wood as a support material for PCM lies in its availability and cost-effectiveness. Furthermore, their mechanical, electrical and thermal properties, such as photoelectric conversion, flame retardancy, and magnetic conductivity, can be easily regulated based on their pores’ nature [35][55].
The incorporation of PCM can be performed on different forms of wood, including solid wood, wood-based composites, and transparent wood. Various tests on PCM-impregnated wood were carried out to determine its characteristics in terms of its heat energy storage capacity, heat stability, heat conductivity, thermal/heat cycling stability, physical (hygroscopic, morphological, leaking) properties, mechanical properties, and chemical and crystalline structure.
Fourier transform infrared spectroscopy (FTIR) tests revealed the PCM-impregnated wood’s chemical components. The type of interaction between the PCM material and wood can be displayed in this result. The interaction is said to occur physically if no new peak appears, indicating that no new chemical component has developed and vice versa if the interactions happen chemically. The PCM material is embedded inside the wood’s porous channels, preventing it from leaking out either in its solid or liquid phase [5][25].
Differential scanning calorimetry (DSC) is used to identify PCM-based wood’s thermal characteristics. The phase change enthalpy is the most reliable indicator for estimating the thermal performance in PCM-based wood. The enthalpy value of PCM-based wood can be improved by subjecting the wood to the delignification process. This fact can be proven by observing the increment of melting enthalpy and solidification enthalpy through the DSC test.
Thermal stability is vital in evaluating PCM-based wood’s practical application value. This can be carried out through a thermogravimetric test by analysing the PCM-based wood’s TG and DTG thermograms. The weight loss rate and residual amount can be used as thermal stability indicators. In this case, a slow weight loss rate with a low amount of residue is favourable. Another method to evaluate the thermal stability of PCM-based wood is by running leakage testing through the melting–impregnation method. The PCM-based wood is heated up to the melting point of PCM. The PCM-based wood is said to own thermal stability if the wood can maintain its shape while effectively trapping or confining the melting PCM inside its pores without leakage. The PCM is trapped inside the porous structure of the wood through capillary action and hydrogen bonding force.
Other than thermal stability, thermal conductivity is another significant indicator of PCM-based wood. As is well-known, the thermal conduction of non-metallic materials is primarily dominated by the thermal vibration of the crystal lattice, namely, phonons [30][50]. The highly conjugated π–π bond in the carbon materials is advantageous in boosting its thermal vibration, thus enhancing the PCM-based wood’s thermal conductivity. Adding materials with electronic structure characteristics may help improve the thermal conductivity of PCM-based wood. This is because this material can facilitate carbon materials’ thermal vibration.
