Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kinza Abbas and Version 2 by Catherine Yang.
The kidney, brain, and retina are highly metabolic organs that require specialized vascular networks to carry out their function. Cohort studies suggest that retinopathy, cerebrovascular disease (CVD), and chronic kidney disease (CKD) frequently coincide. The frequently concurrent prevalence of retinopathy, CVD, and CKD suggests a common basis for pathology.
- small vessel disease
- cerebrovascular disease
- stroke
- retinopathy
- chronic kidney disease
1. Embryology and Genetics
The retina in the optic cup develops from the diencephalon, which is part of the prosencephalon or forebrain. The shared origin and similar development pattern of vasculature by vasculogenesis and angiogenesis may be the cause of linked pathology in the brain and retina [1][2][34,35].
The development of the vasculature supplying the brain begins with the formation of six pairs of primitive branchial arch arteries [3][4][36,37]. The combination of the third branchial arch arteries and the distal segment of the paired dorsal aortae forms the internal carotid artery, which then branches into anterior and posterior divisions [4][37]. The anterior division branches into primitive ophthalmic and olfactory arteries, later giving rise to the anterior cerebral, middle cerebral, and anterior choroidal arteries [5][38]. In contrast, the posterior division gives rise to the posterior cerebral and posterior choroidal arteries [4][37].
The hyaloid artery arises from the primitive dorsal ophthalmic artery and supplies the developing inner retina during its maturation, while the vasculogenesis in the choroid epithelium provides oxygen to the outer avascular retinal layer through diffusion [3][6][7][36,39,40]. The angiogenesis in the retina occurs at the late gestation stage by retinal vasculature, which develops as hyaloid vessels regress [3][6][7][8][9][10][36,39,40,41,42,43]. The vascular layer expands and increases in density following the spread of pigmentation in the retinal epithelium and the production of inductive signals from differentiated retinal pigmental epithelium [11][12][13][14][44,45,46,47].
Similar shared anatomical structures such as fenestrations and regulatory processes, such as the Renin Angiotensin Aldosterone System, may be due to parallel development of glomerular endothelium by vasculogenesis [2][35]. The retina and kidney also share many developmental pathways, abnormalities, and mutations in molecular pathways, which manifest through features in both organs [15][48].
Furthermore, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a rare genetic disorder that primarily affects small vessels in the brain with renal impairment, suggesting a common pathogenetic mechanism of renal and brain lesions in this disease [16][49]. Retinal axonal loss in CADASIL leads to thinning of the retinal nerve fiber length, retinal vein occlusion, macular edema, and retinal venous disease have also been reported among CADASIL patients [17][50]. Another autosomal dominant genetic disorder that links these organs is retinal vasculopathy with cerebral leukodystrophy and systemic manifestations (RVCL-S), which presents with cerebroretinal vasculopathy (CRV), WMHs, hereditary vascular retinopathy (HVR), and hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS) [18][51]. In addition, the growing evidence for the genetic basis of SVD, stemming from MRI studies of WMH, estimate heritability to range between 55–75% [18][51]. Monogenic SVDs, such as COL4A1/A2-related angiopathies and retinal vasculopathy with cerebral leukodystrophy (RVCL), demonstrate cerebral, ocular, and renal abnormalities, implying that patients with monogenic CSVD have concomitant retinopathy and kidney damage [19][52]. These provide further indication of shared genetic and developmental similarities in the brain, kidney, and retina.
2. Cardiovascular Physiology
The frictional force of blood in the vessels, otherwise known as wall shear stress, is counteracted by tension and stretch in the vessel wall. These strains and stretch forces exert a vaso-protective role by providing hemostatic balance. Ito et al. proposed the “strain vessel hypothesis” that detailed the concept that high tone vessels evolutionarily developed to maintain perfusion of the brain and kidney. Although they are exposed to high blood pressure, vascular damage from prolonged hemodynamic stress and a perturbation to this biochemical balance results in either physiological adaptation or disease of the vessel walls [20][21][22][1,53,54]. Thus, common vascular risk factors such as high blood pressure and diabetes mellitus may cause damage to these strain vessels in parallel in the brain and kidney [22][54]. Non-traditional CKD-related risk factors, such as chronic inflammation, endothelial dysfunction, and uremic toxins, can promote cerebrovascular injury by triggering vascular injury and endothelial dysfunction [23][55].
3. Pathology
The numerous anatomical and functional similarities in the brain, retina, and kidney provide a basis for common pathology. The blood–brain barrier is formed by endothelial cells of the capillary wall with tight junctions, astrocytes with projections sheathing the capillary, and pericytes embedded in the capillary basement membrane [24][56]. Like the brain, the retina possesses barrier circulation; the blood–retina barrier is arranged as inner and outer barriers [25][57]. The inner retinal barrier is comprised of endothelial cells with tight junctions and is surrounded by glial cells to maintain retinal homeostasis [25][26][57,58].
Similar to the glomerular filtration barrier, the outer retinal barrier formed at the retinal pigment epithelial cell layers functions to regulate the movement of solutes and nutrients from the choroid to the sub-retinal space [25][27][57,59]. The arrangement of the choriocapillaris, Bruch’s membrane, and retinal pigment epithelial interface are homologous to the endothelium, glomerular basement membrane, and podocytes in the glomerulus (Figure 1) [28][60]. Physiologically, the glomerulus and choriocapillaris are both fenestrated, while the podocytes and retinal pigment epithelial cells both function as metabolic barriers, actively mediating the exchange of molecules [27][29][59,61]. Additionally, Bruch’s membrane and the glomerular basement membrane both contain a network of a3, a4, and a5 type IV collagen chains [27][30][59,62].
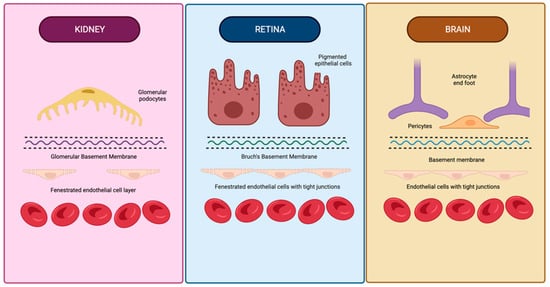
Figure 1. Similarities in anatomical structure of vascular barriers in the brain, retina, and kidneys. The vascular barriers of the brain, retina, and kidneys have many structural similarities, including basement membranes containing type IV collagen, an outer layer with analogous projections (glomerular podocyte foot processes, retinal epithelial cell projections, and astrocyte foot processes), and a fenestrated endothelial cell layer in the glomerulus and retina. Created using BioRender.com on 26 August 2022.
The anatomical and compositional similarities manifest as simultaneous retinopathy and nephropathy in membranoproliferative glomerulonephritis type II and Alport syndrome [31][32][33][34][63,64,65,66]. The simultaneous pathology and appearance of clinical symptoms in chronic cardiovascular diseases, hypertension, and diabetes mellitus further provide evidence of a link between these organs. Hemodynamic similarities of vascular beds of the brain and kidney are generally noted as the shared mechanism linking cerebral and renal vascular dysfunction biomarkers. The kidney and brain are uniquely low-resistance end-organs exposed to consistent high-volume blood flow [22][54].
Thus, despite differences in location and responses to injury, concurrent changes are seen in the micro-vasculature of these organs under pathological conditions, which the reserchers hypothesize to be due to their common embryological, functional, histological, and genetic basis (Figure 2).
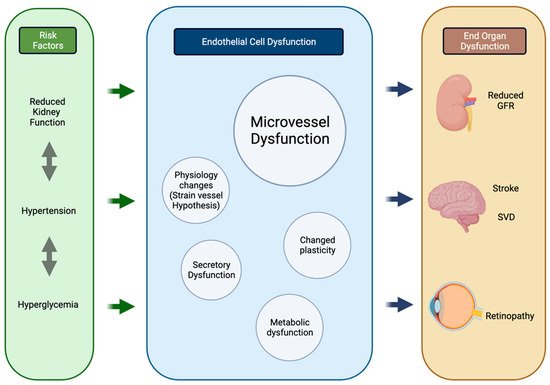
Figure 2. Process of parallel onset end-organ dysfunction. Flowchart demonstrating the interactions between shared systemic risk factors, resulting in endothelial cell changes and end-organ manifestations in the kidneys, brain, and retina. Created using BioRender.com. GFR glomerular filtration rate, SVD small vessel disease. Created using BioRender.com on 25 August 2022.
