Small noncoding RNAs (sRNA) appear to play a key role in extracellular vesicle (EV)-mediated information transfer. Within the vesicular envelope, RNAs are well protected from degradation and can be shuttled between individuals from one and the same species and beyond. Various communication routes have been discovered such as mother-infant-interaction via breast milk, diverse host-pathogen-relations, and dietary uptake of food derived EVs, proving that EV-mediated inter-kingdom regulation is more than a random event.
- extracellular vesicles
- cross-kingdom RNA interference
- host-pathogen-interaction
1. Introduction
After the observation that EV-mediated information transfer is not limited to one organism, species or kingdoms, the one central question of EV research became, “Why can EVs overcome kingdom boundaries?” Investigations on EV-mediated regulation processes, from mother–infant to host–pathogen interaction, might elucidate this query (Table 1).
Table 1. EV mediated regulation processes.
|
EV Mediated Regulations |
Examples |
References |
|
|
Inter-individual regulation |
mother ↔ foetus |
[1] |
|
|
mother → infant regulation |
|||
|
elevated fungal virulence |
|||
|
elevated bacterial virulence/drug resistance within the same species |
|||
|
Interspecies regulation |
dietary uptake, e.g., bovine milk → other mammals |
||
|
pathogen-host interactions, e.g., helminth ↔ animal host |
|||
|
elevated bacterial virulence/drug resistance beyond species boundaries |
|||
|
archaeal antimicrobial proteins inhibit growth of other archaea |
|||
|
archaeal DNA tranfer |
|||
|
Inter-kingdom regulation |
pathogen–host interactions: |
plant ↔ fungus |
|
|
animal ↔ fungus |
|||
|
bacteria ↔ animal |
[9][10][27][28][29][30][31][32][33] |
||
|
dietary uptake, e.g., rice → mammal |
|||
|
archaeal antimicrobial proteins inhibit bacterial growth |
|||
|
EV Mediated Regulations |
Examples |
References |
|
|
Inter-individual regulation |
mother ↔ foetus |
[1] |
|
|
mother → infant regulation |
|||
|
elevated fungal virulence |
|||
|
elevated bacterial virulence/drug resistance within the same species |
|||
|
Interspecies regulation |
dietary uptake, e.g., bovine milk → other mammals |
||
|
pathogen-host interactions, e.g., helminth ↔ animal host |
|||
|
elevated bacterial virulence/drug resistance beyond species boundaries |
|||
|
archaeal antimicrobial proteins inhibit growth of other archaea |
|||
|
archaeal DNA tranfer |
|||
|
Inter-kingdom regulation |
pathogen–host interactions: |
plant ↔ fungus |
|
|
animal ↔ fungus |
|||
|
bacteria ↔ animal |
[9][10][27][28][29][30][31][32][33] |
||
|
dietary uptake, e.g., rice → mammal |
|||
|
archaeal antimicrobial proteins inhibit bacterial growth |
|||
2. Inter-Individual, Interspecies, and Inter-Kingdom Regulation
In addition to mitogenic lipids and signaling proteins, sRNAs are considered to be crucial regulatory elements in EV-mediated (inter-kingdom) communication[36]. They are able to manipulate various biological processes, such as cell growth, differentiation, development, metabolism, and apoptosis[37][38]. Stability and absorption of sRNA are obviously critical aspects of bioavailability for recipient organisms or cells. In contrast to traditional persuasions on the stability of extracellular RNA, a few studies have shown surprisingly high pH-, temperature-, and RNase-resistances for sRNA in mammalian body fluids[13][39][40][41][42][43] , as well as for plant sRNAs[44] [45][46][47][48]. The vesicular envelope of EVs is thought to be decisive for the enhanced sRNA stability. This assumption is strongly underlined by the fact that severe losses of sRNA are detectable after pasteurization and homogenization or after ultrasonic exosome depletion of bovine milk[2][49][50]. Furthermore, the envelope also provides a vehicle for cellular uptake of the cargo, not only in the intestine[2][49][12][41][51][52][53].
Since EVs have been found in the milk of distinct mammals, such as pork, cow, or human, increasing numbers of inter-individual and interspecies regulation processes are being assumed highly probable[2][41][54][55][56][57]. Moreover, increased serum levels of bovine milk specific sRNA were detected in humans after consumption of cow´s milk[11]. Until today, we are lacking reliable studies on physiological or pathological effects of ingested EVs on humans, while a broad range of such effects is conceivable. This assumption is supported by investigations that have shown that a breastfed infant profits from ingested milk-derived sRNAs by elevated T-cell levels and enhanced differentiation of B cells[38][2][41][54].
Although there has been previous evidence for inter-kingdom regulation mediated by sRNAs[58][59][60][61], the study by Zhang et al., 2012 was somehow paradigm shifting. Their finding, that the dietary uptake of a particular plant-derived micro RNA can measurably affect the metabolism of a mammal[62], quickly ignited increased interest in this field.
Probably, fungal cells send EVs in order to downregulate host immune response. Observations in both human–fungus and plant–fungus interactions suggest fungal virulence to be strongly enhanced by inter-kingdom RNA interference, enabled by sRNA containing EVs[26][5][22][24][63]. Conversely, plants send sRNA to silence fungal virulence genes, which has recently also been related to EVs[19][20][21][64][63][65].
In the area of difficult-to-treat infections, OMVs play a major role in drug resistance because they transfer resistance genes (DNA) between bacteria, even of different origin[7] . Many OMVs from pathogenic bacteria were found to have surface proteins, which can readily interact with mammalian host cells. These interaction mechanisms make OMVs a pivotal element of trans-kingdom and host-cell communication by letting them interact in a highly specific manner[66]. OMVs have been shown to carry PAMPs, including lipopolysaccharides, and can transfer other virulence associated factors[67]. These factors can trigger strong immune responses in host cells, while OMVs act as immunomodulators, for example, by leading to expression of receptors on macrophages to specifically recognize the pathogen[68]. As OMVs can help pathogenic bacteria to persist attack by the mammalian immune system, they strongly contribute to the cause of infectious disease[10][27]. Prokaryotic pathogens such as Bacillus anthracis Cohn[28], Helicobacter pylori (Marshall) Goodwin[29], Neisseria gonorrhoeae (Zopf) Trevisan[30], Pseudomonas aeruginosa (Schroeter) Migula[31], and Streptococcus pneumoniae (Klein) Chester[32], as well as eukarytotic pathogens such as Leishmania spp. Ross[69], Plasmodium spp. Marchiafava et Celli[70], and Trichomonas vaginalis Donné[71]similarly send EVs to increase their contagiousness[33][72][73][74]. This phenomenon is not limited to unicellular organisms, since helminths also modulate host immunity, as Heligosomoides polygyrus Dujardin[13] and Dicrocoelium dendriticum Rudolphi[75].
Overall, EVs appear to be potent agents in regulation processes, crossing not only the borders of species but rather of kingdoms or even empires. Therefore, they enhance an arms race in host–pathogen interaction[64][23]. But do exosomes also facilitate intercellular communication beyond the animal kingdom? Especially host–pathogen interactions imply the possibility of host-host and pathogen-pathogen signaling, intended to improve the chance of survival on each side (Figure 1). A better understanding of host-pathogen interactions can elucidate unknown mechanisms, and therefore future targets, improving therapies of infectious diseases.
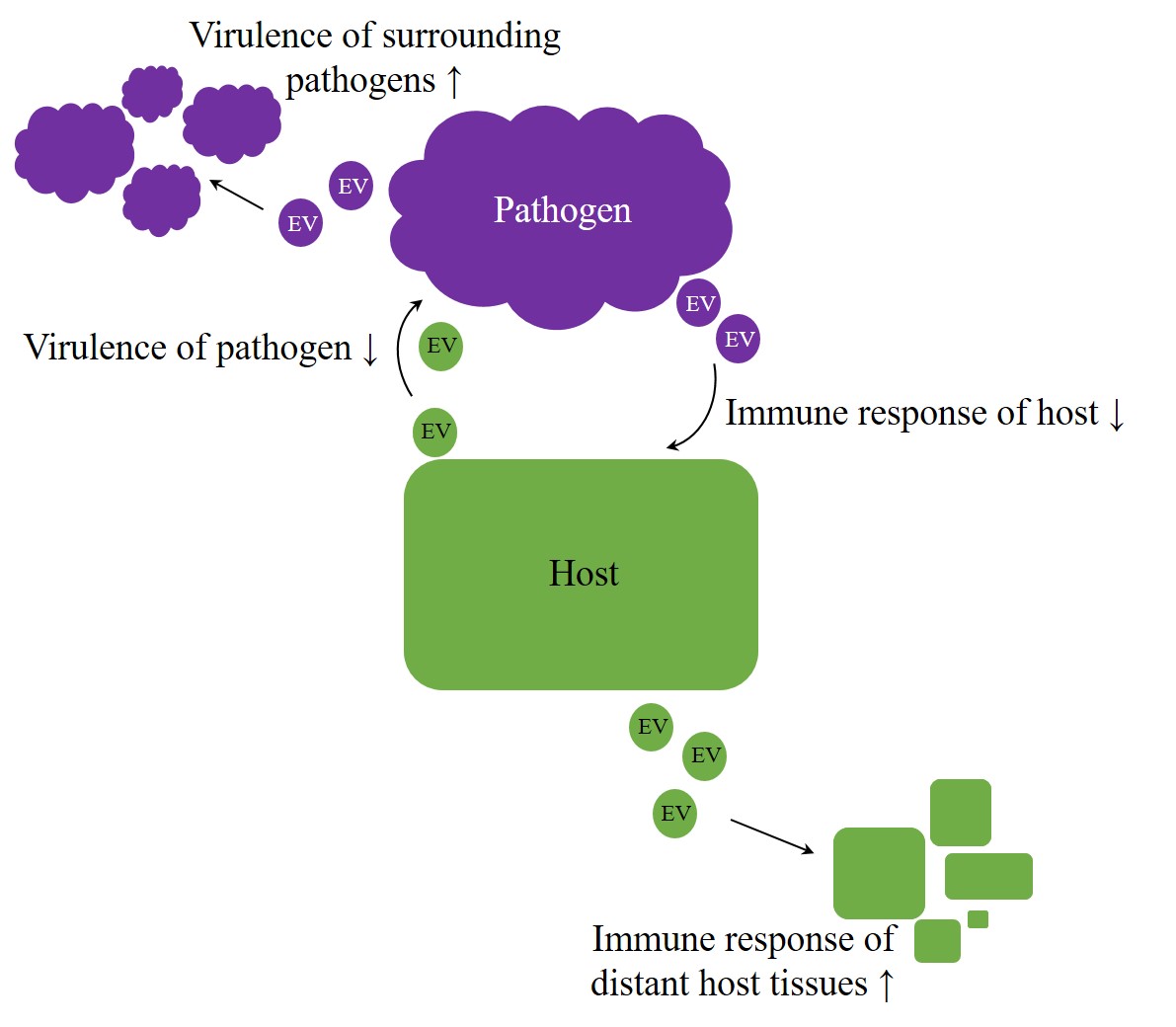
Figure 1. Arms race in host–pathogen interaction. Irrespective of kingdom boundaries, the genuine role of extracellular vesicles (EVs) appears to be bilateral. On the one hand, they were proven to have protective properties, but, on the other hand, they also appear to contribute to the achievement of inherent aims, like enhancing virulence on pathogens side or improving host´s immunity.
References
- Mancy Tong; L.W. Chamley; Placental Extracellular Vesicles and Feto-Maternal Communication. Cold Spring Harbor Perspectives in Medicine 2015, 5, a023028-a023028, 10.1101/cshperspect.a023028.
- Janos Zempleni; Ana Aguilar-Lozano; Mahrou Sadri; Sonal Sukreet; Sonia Manca; Di Wu; Fang Zhou; Ezra Mutai; Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants.. The Journal of Nutrition 2016, 147, 3-10, 10.3945/jn.116.238949.
- Cecilia Lässer; Vesta Seyed Alikhani; Karin Ekström; Maria Eldh; Patricia Torregrosa Paredes; Apostolos Bossios; Margareta Sjöstrand; Susanne Gabrielsson; Jan Lötvall; Hadi Valadi; et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. Journal of Translational Medicine 2011, 9, 9-9, 10.1186/1479-5876-9-9.
- Marijke I. Zonneveld; Alain R. Brisson; Martijn J. C. Van Herwijnen; Sisareuth Tan; Chris H. A. Van De Lest; Frank A. Redegeld; Johan Garssen; Marca H. M. Wauben; Esther N. M. Nolte-'t Hoen; Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. Journal of Extracellular Vesicles 2014, 3, 1, 10.3402/jev.v3.24215.
- Marcio L. Rodrigues; Leonardo Nimrichter; Debora L. Oliveira; Joshua D. Nosanchuk; Arturo Casadevall; Vesicular Trans-Cell Wall Transport in Fungi: A Mechanism for the Delivery of Virulence-Associated Macromolecules?. Lipid Insights 2008, 2, 27-40, 10.4137/lpi.s1000.
- Ewa Bielska; Marta Arch Sisquella; Maha Aldeieg; Charlotte Birch; Eloise J. O’Donoghue; Robin C. May; Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii.. Nature Communications 2018, 9, 1556, 10.1038/s41467-018-03991-6.
- Jennifer M. Bomberger; Daniel P. MacEachran; Bonita A. Coutermarsh; Siying Ye; George A. O'toole; Bruce A. Stanton; Long-Distance Delivery of Bacterial Virulence Factors by Pseudomonas aeruginosa Outer Membrane Vesicles. PLOS Pathogens 2009, 5, e1000382, 10.1371/journal.ppat.1000382.
- Madhab Kumar Chattopadhyay; Medicharla Venkata Jagannadham; Vesicles-mediated resistance to antibiotics in bacteria. Frontiers in Cellular and Infection Microbiology 2015, 6, 758, 10.3389/fmicb.2015.00758.
- Arif Tasleem Jan; Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Frontiers in Microbiology 2017, 8, 1053, 10.3389/fmicb.2017.01053.
- Terri N. Ellis; Meta J. Kuehn; Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiology and Molecular Biology Reviews 2010, 74, 81-94, 10.1128/MMBR.00031-09.
- Scott R. Baier; Christopher Nguyen; Fang Xie; Jennifer R. Wood; Janos Zempleni; MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers.. The Journal of Nutrition 2014, 144, 1495-500, 10.3945/jn.114.196436.
- Tovah Wolf; Scott R Baier; Janos Zempleni; The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells.. The Journal of Nutrition 2015, 145, 2201-2206, 10.3945/jn.115.218586.
- Amy H. Buck; Gillian Coakley; Fabio Simbari; Henry J. McSorley; Juan F. Quintana; Thierry Le Bihan; Sujai Kumar; Cei Abreu-Goodger; Marissa Lear; Yvonne Harcus; et al.Alessandro CeroniSimon A. BabayanMark BlaxterAlasdair IvensRick M. Maizels Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nature Communications 2014, 5, 5488, 10.1038/ncomms6488.
- Sima Yaron; Glynis L. Kolling; Lee Simon; Karl R. Matthews; Vesicle-Mediated Transfer of Virulence Genes from Escherichia coli O157:H7 to Other Enteric Bacteria. Applied and Environmental Microbiology 2000, 66, 4414-4420, 10.1128/aem.66.10.4414-4420.2000.
- David Prangishvili; Ingelore Holz; Evelyn Stieger; Stephan Nickell; Jakob K. Kristjansson; Wolfram Zillig; Sulfolobicins, Specific Proteinaceous Toxins Produced by Strains of the Extremely Thermophilic Archaeal Genus Sulfolobus. Journal of Bacteriology 2000, 182, 2985-2988, 10.1128/jb.182.10.2985-2988.2000.
- Albert F. Ellen; Olha V. Rohulya; Fabrizia Fusetti; Michaela Wagner; Sonja-Verena Albers; Arnold J. M. Driessen; The Sulfolobicin Genes of Sulfolobus acidocaldariusEncode Novel Antimicrobial Proteins ▿ †. Journal of Bacteriology 2011, 193, 4380-4387, 10.1128/JB.05028-11.
- Marie Gaudin; Emilie Gauliard; Pascal Lenormand; Evelyne Marguet; Ludivine Houel‐Renault; Stefan Schouten; Patrick Forterre; Hyperthermophilic archaea produce membrane vesicles that can transfer DNA. Environmental Microbiology Reports 2012, 5, 109-116, 10.1111/j.1758-2229.2012.00348.x.
- Marie Gaudin; Mart Krupovič; Evelyne Marguet; Emilie Gauliard; Eric Le Cam; Jacques Oberto; Patrick Forterre; Virginija Cvirkaite-Krupovic; Virginija Cvirkaitė-Krupovič; Virginija Cvirkaite‐Krupovic; et al. Extracellular membrane vesicles harbouring viral genomes. Environmental Microbiology 2013, 16, 1167-1175, 10.1111/1462-2920.12235.
- Brian D. Rutter; Roger W. Innes; Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins.. Plant Physiology 2016, 173, 728-741, 10.1104/pp.16.01253.
- Mariana Regente; Marcela Pinedo; Hélène San Clemente; Thierry Balliau; Elisabeth Jamet; Laura De La Canal; Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. Journal of Experimental Botany 2017, 68, 5485-5495, 10.1093/jxb/erx355.
- Qiang Cai; Lulu Qiao; Ming Wang; Baoye He; Feng-Mao Lin; Jared Palmquist; Sienna-Da Huang; Hailing Jin; Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126-1129, 10.1126/science.aar4142.
- Arne Weiberg; Ming Wang; Feng-Mao Lin; Hongwei Zhao; Zhihong Zhang; Isgouhi Kaloshian; Hsien-Da Huang; Hailing Jin; Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways.. Science 2013, 342, 118-23, 10.1126/science.1239705.
- Arne Weiberg; Hailing Jin; Small RNAs--the secret agents in the plant-pathogen interactions.. Current Opinion in Plant Biology 2015, 26, 87-94, 10.1016/j.pbi.2015.05.033.
- Ming Wang; Arne Weiberg; Exequiel Dellota; Daniel Yamane; Hailing Jin; Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biology 2017, 14, 421-428, 10.1080/15476286.2017.1291112.
- Gabriele Vargas; Juliana D. B. Rocha; Debora Leite Oliveira; Priscila Costa Albuquerque; Susana Frases; Suelen S. Santos; Joshua Daniel Nosanchuk; Andre Marco Oliveira Gomes; Lia C. A. S. Medeiros; Kildare Miranda; et al.Tiago J. P. SobreiraErnesto S. NakayasuEmma A. ArigiArturo CasadevallAllan J. GuimarãesMarcio L. RodriguesCelio Geraldo Freire-De-LimaIgor C. AlmeidaLeonardo NimrichterA. M. O. GomesL. C. Soares MedeirosK. R. MirandaM. L. RodriguesC. G. Freire‐De‐LimaMarcio RodriguesCélio Geraldo Freire-De-Lima Compositional and immunobiological analyses of extracellular vesicles released byCandida albicans. Cellular Microbiology 2014, 17, 389-407, 10.1111/cmi.12374.
- Ulf Gehrmann; Khaleda Rahman Qazi; Catharina Johansson; Kjell Hultenby; Maria Karlsson; Lena Lundeberg; Susanne Gabrielsson; Annika Scheynius; Nanovesicles from Malassezia sympodialis and Host Exosomes Induce Cytokine Responses – Novel Mechanisms for Host-Microbe Interactions in Atopic Eczema. PLOS ONE 2011, 6, e21480, 10.1371/journal.pone.0021480.
- Ian A. Macdonald; Meta J. Kuehn; Stress-Induced Outer Membrane Vesicle Production by Pseudomonas aeruginosa. Journal of Bacteriology 2013, 195, 2971-2981, 10.1128/JB.02267-12.
- Johanna Rivera; Radames J. B. Cordero; Antonio S. Nakouzi; Susana Frases; André Nicola; Arturo Casadevall; Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proceedings of the National Academy of Sciences 2010, 107, 19002-19007, 10.1073/pnas.1008843107.
- Maria Kaparakis; Lynne Turnbull; Leticia Carneiro; Stephen Firth; Harold A. Coleman; Helena C. Parkington; Lionel Le Bourhis; Abdulgader Karrar; Jérôme Viala; Johnson Mak; et al.Melanie L. HuttonJohn K. DaviesPeter J. CrackPaul J. HertzogDana J. PhilpottStephen E. GirardinCynthia B. WhitchurchRichard L. Ferrero Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cellular Microbiology 2010, 12, 372-385, 10.1111/j.1462-5822.2009.01404.x.
- Pankaj Deo; Seong H. Chow; Iain D. Hay; Oded Kleifeld; Adam Costin; Kirstin D. Elgass; Jhih-Hang Jiang; Georg Ramm; Kipros Gabriel; Gordon Dougan; et al.Trevor LithgowEva HeinzThomas Naderer Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLOS Pathogens 2018, 14, e1006945, 10.1371/journal.ppat.1006945.
- Katja Koeppen; Thomas H. Hampton; Michael Jarek; Maren Scharfe; Scott A. Gerber; Daniel W. Mielcarz; Elora G. Demers; Emily L. Dolben; John H. Hammond; Deborah A. Hogan; et al.Bruce A. Stanton A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. PLOS Pathogens 2016, 12, e1005672, 10.1371/journal.ppat.1005672.
- Mario Codemo; Sandra Muschiol; Federico Iovino; Priyanka Nannapaneni; Laura Plant; Sun Nyunt Wai; Birgitta Henriques-Normark; Immunomodulatory Effects of Pneumococcal Extracellular Vesicles on Cellular and Humoral Host Defenses. mBio 2018, 9, e00559-18-18, 10.1128/mBio.00559-18.
- Ivan K. H. Poon; Christopher D. Gregory; Maria Kaparakis-Liaskos; Editorial: The Immunomodulatory Properties of Extracellular Vesicles From Pathogens, Immune Cells, and Non-immune Cells. Frontiers in Immunology 2018, 9, 3024, 10.3389/fimmu.2018.03024.
- Kurataka Otsuka; Yusuke Yamamoto; Ryosuke Matsuoka; Takahiro Ochiya; Maintaining good miRNAs in the body keeps the doctor away?: Perspectives on the relationship between food-derived natural products and microRNAs in relation to exosomes/extracellular vesicles. Molecular Nutrition & Food Research 2017, 62, 1700080, 10.1002/mnfr.201700080.
- Lin Zhang; Dongxia Hou; Xi Chen; Donghai Li; Lingyun Zhu; Yujing Zhang; Jing Li; Zhen Bian; Xiangying Liang; Xing Cai; et al.Yuan YinCheng WangTianfu ZhangDihan ZhuDianmu ZhangJie XuQun ChenYi BaJing LiuQiang WangJianqun ChenJin WangMeng WangQipeng ZhangJunfeng ZhangKe ZenChen-Yu Zhang Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Research 2011, 22, 273-274, 10.1038/cr.2011.174.
- Michel Record; Exosome-like nanoparticles from food: protective nanoshuttles for bioactive cargo.. Molecular Therapy 2013, 21, 1294-6, 10.1038/mt.2013.130.
- David P Bartel; MicroRNAs. Cell 2004, 116, 281-297, 10.1016/s0092-8674(04)00045-5.
- Mengxi Jiang; Xiaolin Sang; Zhi Hong; Beyond nutrients: Food-derived microRNAs provide cross-kingdom regulation. BioEssays 2012, 34, 280-284, 10.1002/bies.201100181.
- Xi Chen; Yi Ba; Lijia Ma; Xing Cai; Yuan Yin; Kehui Wang; Jigang Guo; Yujing Zhang; Jiangning Chen; Xing Guo; et al.Qibin LiXiaoying LiWenjing WangYan ZhangJin WangXueyuan JiangYang XiangChen XuPingping ZhengJuanbin ZhangRuiqiang LiHongjie ZhangXiaobin ShangTing GongGuang NingJun WangKe ZenJunfeng ZhangChen-Yu Zhang Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Research 2008, 18, 997-1006, 10.1038/cr.2008.282.
- Patrick S. Mitchell; Rachael K. Parkin; Evan M. Kroh; Brian R. Fritz; Stacia K. Wyman; Era L. Pogosova-Agadjanyan; Amelia Peterson; Jennifer Noteboom; Kathy C. O'briant; April Allen; et al.Daniel W. LinNicole UrbanCharles W. DrescherBeatrice S. KnudsenDerek L. StirewaltRobert GentlemanRobert L. VessellaPeter S. NelsonDaniel B. MartinMuneesh Tewari Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences 2008, 105, 10513-10518, 10.1073/pnas.0804549105.
- Nobuyoshi Kosaka; Hirohisa Izumi; Kazunori Sekine; Takahiro Ochiya; microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7-7, 10.1186/1758-907X-1-7.
- H. Izumi; N. Kosaka; T. Shimizu; K. Sekine; T. Ochiya; M. Takase; Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. Journal of Dairy Science 2012, 95, 4831-4841, 10.3168/jds.2012-5489.
- Mohammed Alsaweed; Peter E. Hartmann; Donna T. Geddes; Foteini Kakulas; MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. International Journal of Environmental Research and Public Health 2015, 12, 13981-14020, 10.3390/ijerph121113981.
- Wenyan Xie; Alexander Weng; Matthias F. Melzig; MicroRNAs as New Bioactive Components in Medicinal Plants. Planta Medica 2016, 82, 1153-1162, 10.1055/s-0042-108450.
- Songwen Ju; Jingyao Mu; Terje Dokland; Xiaoying Zhuang; Qilong Wang; Hong Jiang; Xiaoyu Xiang; Zhong-Bin Deng; Baomei Wang; Lifeng Zhang; et al.Mary RothRuth WeltiJames MobleyYan JunDonald MillerHuang-Ge Zhang Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Molecular Therapy 2013, 21, 1345-1357, 10.1038/mt.2013.64.
- Baomei Wang; Xiaoying Zhuang; Zhong-Bin Deng; Hong Jiang; Jingyao Mu; Qilong Wang; Xiaoyu Xiang; Haixun Guo; Lifeng Zhang; Gerald Dryden; et al.Jun YanDonald MillerHuang-Ge Zhang Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Molecular Therapy 2014, 22, 522-534, 10.1038/mt.2013.190.
- Junjie Li; Zhiyong Yang; Bin Yu; Jun Liu; Xuemei Chen; Methylation protects miRNAs and siRNAs from a 3'-end uridylation activity in Arabidopsis.. Current Biology 2005, 15, 1501-7, 10.1016/j.cub.2005.07.029.
- Wenyan Xie; Matthias F. Melzig; The Stability of Medicinal Plant microRNAs in the Herb Preparation Process. Molecules 2018, 23, 919, 10.3390/molecules23040919.
- Scott R. Baier; Christopher Nguyen; Fang Xie; Jennifer R. Wood; Janos Zempleni; MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers.. The Journal of Nutrition 2014, 144, 1495-1500, 10.3945/jn.114.196436.
- Katherine M. Howard; Rio Jati Kusuma; Scott R. Baier; Taylor Friemel; Laura Markham; Jairam Vanamala; Janos Zempleni; And Janos Zempleni; Loss of miRNAs during Processing and Storage of Cow’s (Bos taurus) Milk. Journal of Agricultural and Food Chemistry 2015, 63, 588-592, 10.1021/jf505526w.
- Cristina Escrevente; Sascha Keller; Peter Altevogt; Júlia Costa; Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108-108, 10.1186/1471-2407-11-108.
- Laura Ann Mulcahy; Ryan Charles Pink; David Raul Francisco Carter; Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles 2014, 3, 1093, 10.3402/jev.v3.24641.
- Radha Munagala; Farrukh Aqil; Jeyaprakash Jeyabalan; Ramesh C. Gupta; Bovine milk-derived exosomes for drug delivery.. Cancer Letters 2015, 371, 48-61, 10.1016/j.canlet.2015.10.020.
- Charlotte Admyre; Sara M. Johansson; Khaleda Rahman Qazi; Jan-Jonas Filén; Riitta Lahesmaa; Mikael Norman; Etienne P. A. Neve; Annika Scheynius; Susanne Gabrielsson; Exosomes with immune modulatory features are present in human breast milk.. The Journal of Immunology 2007, 179, 1969-1978, 10.4049/jimmunol.179.3.1969.
- Xi Chen; Chao Gao; Haijin Li; Lei Huang; Qi Sun; Yanye Dong; Chunliang Tian; Shengpu Gao; Hailin Dong; Danping Guan; et al.Xiaoyun HuShujian ZhaoLiang LiLin ZhuQiao YanJunfeng ZhangKe ZenChen-Yu Zhang Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Research 2010, 20, 1128-1137, 10.1038/cr.2010.80.
- Taketoshi Hata; Kosuke Murakami; Hajime Nakatani; Yasunari Yamamoto; Tsukasa Matsuda; Naohito Aoki; Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochemical and Biophysical Research Communications 2010, 396, 528-533, 10.1016/j.bbrc.2010.04.135.
- Ting Chen; Mei-Ying Xie; Jia-Jie Sun; Rui-Song Ye; Xiao Cheng; Rui-Ping Sun; Li-Min Wei; Meng Li; De-Lin Lin; Qing-Yan Jiang; et al.Qian-Yun XiYong-Liang Zhang Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Scientific Reports 2016, 6, 33862, 10.1038/srep33862.
- Lisa Timmons; Andrew Fire; Specific interference by ingested dsRNA. Nature 1998, 395, 854-854, 10.1038/27579.
- Phillip A. Newmark; Peter W. Reddien; Francesc Cebria; Alejandro Sánchez Alvarado; Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proceedings of the National Academy of Sciences 2003, 100, 11861-11865, 10.1073/pnas.1834205100.
- Z. Issa; W.N. Grant; S. Stasiuk; C.B. Shoemaker; Development of methods for RNA interference in the sheep gastrointestinal parasite, Trichostrongylus colubriformis. International Journal for Parasitology 2005, 35, 935-940, 10.1016/j.ijpara.2005.06.001.
- Olle Terenius; Alexie Papanicolaou; Jennie S. Garbutt; Ioannis Eleftherianos; Hanneke Huvenne; Sriramana Kanginakudru; Merete Albrechtsen; Chunju An; Jean-Luc Aymeric; Andrea Barthel; et al.Piotr BebasKavita BitraAlejandra BravoFrançois ChevalierDerek P. CollingeCristina M. CravaRuud A. De MaagdBernard DuvicMartin ErlandsonIngrid FayeGabriella FelföldiHaruhiko FujiwaraRyo FutahashiArchana S. GandheHeather S. GatehouseLaurence N. GatehouseJadwiga M. GiebultowiczIsabel GómezCornelis J.P. GrimmelikhuijzenAstrid T. GrootFrank HauserDavid G. HeckelDwayne D. HegedusSteven HrycajLihua HuangJ. Joe HullKostas IatrouMasatoshi IgaMichael R. KanostJoanna KotwicaChangyou LiJianghong LiJisheng LiuMagnus LundmarkShogo MatsumotoMartina Meyering-VosPeter J. MillichapAntónia MonteiroNirotpal MrinalTeruyuki NiimiDaniela NowaraAtsushi OhnishiVicencio OostraKatsuhisa OzakiMaria PapakonstantinouAleksandar PopadicManchikatla V. RajamSuzanne SaenkoRobert M. SimpsonMario SoberónMichael R. StrandShuichiro TomitaUmut ToprakPing WangChoon Wei WeeSteven WhyardWenqing ZhangJavaregowda NagarajuRichard H. Ffrench-ConstantSalvador HerreroKarl GordonLuc SweversGuy Smagghe RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. Journal of Insect Physiology 2011, 57, 231-245, 10.1016/j.jinsphys.2010.11.006.
- Lin Zhang; Dongxia Hou; Xi Chen; Donghai Li; Lingyun Zhu; Yujing Zhang; Jing Li; Zhen Bian; Xiangying Liang; Xing Cai; et al.Yuan YinCheng WangTianfu ZhangDihan ZhuDianmu ZhangJie XuQun ChenYi BaJing LiuQiang WangJianqun ChenJin WangMeng WangQipeng ZhangJunfeng ZhangKe ZenChen-Yu Zhang Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Research 2011, 22, 107-126, 10.1038/cr.2011.158.
- Tao Zhang; Yun-Long Zhao; Jian-Hua Zhao; Sheng Wang; Yun Jin; Zhong-Qi Chen; Yuan-Yuan Fang; Chen-Lei Hua; Shou-Wei Ding; Hui-Shan Guo; et al. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nature Plants 2016, 2, 16153, 10.1038/nplants.2016.153.
- Ming Wang; Arne Weiberg; Feng-Mao Lin; Bart P. H. J. Thomma; Hsien-Da Huang; Hailing Jin; Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nature Plants 2016, 2, 16151-16151, 10.1038/nplants.2016.151.
- Claudia Castillo-González; Xiuren Zhang; The Trojan Horse of the Plant Kingdom. Cell Host & Microbe 2018, 24, 1-3, 10.1016/j.chom.2018.06.015.
- Sapna Jain; Jonathan Pillai; Bacterial membrane vesicles as novel nanosystems for drug delivery. International Journal of Nanomedicine 2017, 12, 6329-6341, 10.2147/IJN.S137368.
- Maria Kaparakis-Liaskos; Richard L. Ferrero; Immune modulation by bacterial outer membrane vesicles. Nature Reviews Immunology 2015, 15, 375-387, 10.1038/nri3837.
- Oh Youn Kim; Bok Sil Hong; Kyong-Su Park; Yae Jin Yoon; Seng Jin Choi; Won Hee Lee; Tae-Young Roh; Jan Lötvall; Yoon-Keun Kim; Yong Song Gho; et al. Immunization with Escherichia coli Outer Membrane Vesicles Protects Bacteria-Induced Lethality via Th1 and Th17 Cell Responses. The Journal of Immunology 2013, 190, 4092-4102, 10.4049/jimmunol.1200742.
- Judith Maxwell Silverman; Joachim Clos; Eva Horakova; Adele Y. Wang; Martina Wiesgigl; Isabelle Kelly; Miriam A. Lynn; W. Robert McMaster; Leonard J. Foster; Megan K. Levings; et al.Neil E. Reiner Leishmania Exosomes Modulate Innate and Adaptive Immune Responses through Effects on Monocytes and Dendritic Cells. The Journal of Immunology 2010, 185, 5011-5022, 10.4049/jimmunol.1000541.
- Ladawan Khowawisetsut; Extracellular Vesicles in Malaria Infection. Siriraj Medical Journal 2019, 71, 89–94, 10.33192/smj.2019.14.
- Olivia Twu; Natalia De Miguel; Gila Lustig; Grant C. Stevens; Ajay A. Vashisht; James A. Wohlschlegel; Patricia J. Johnson; Trichomonas vaginalis Exosomes Deliver Cargo to Host Cells and Mediate Host∶Parasite Interactions. PLOS Pathogens 2013, 9, e1003482, 10.1371/journal.ppat.1003482.
- Antonio Marcilla; Lorena Martin-Jaular; Maria Trelis; Armando De Menezes-Neto; Antonio Osuna; Dolores Bernal; Carmen Fernandez-Becerra; Igor C. Almeida; Hernando A. Del Portillo; Extracellular vesicles in parasitic diseases. Journal of Extracellular Vesicles 2014, 3, 25040, 10.3402/jev.v3.25040.
- Marije E. Kuipers; Cornelis H. Hokke; Hermelijn H. Smits; Esther N. M. Nolte-‘T Hoen; Pathogen-Derived Extracellular Vesicle-Associated Molecules That Affect the Host Immune System: An Overview. Frontiers in Microbiology 2018, 9, 2182, 10.3389/fmicb.2018.02182.
- Gebeyaw Getnet Mekonnen; Mark Pearson; Alex Loukas; Javier Sotillo; Extracellular vesicles from parasitic helminths and their potential utility as vaccines. Expert Review of Vaccines 2018, 17, 197-205, 10.1080/14760584.2018.1431125.
- Dolores Bernal; Maria Trelis; Sergio Montaner; Fernando Cantalapiedra; Alicia Galiano; Michael Hackenberg; Antonio Marcilla; Sergio Montaner Tarbes; Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. Journal of Proteomics 2014, 105, 232-241, 10.1016/j.jprot.2014.02.012.
- Dolores Bernal; Maria Trelis; Sergio Montaner; Fernando Cantalapiedra; Alicia Galiano; Michael Hackenberg; Antonio Marcilla; Sergio Montaner Tarbes; Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. Journal of Proteomics 2014, 105, 232-241, 10.1016/j.jprot.2014.02.012.
