Current treatment paradigms for end-stage dilated cardiomyopathy (DCM) in children include heart transplantation and mechanical support devices. However, waitlist mortality, shortage of smaller donors, time-limited durability of grafts, and thrombo-hemorrhagic events affect long-term outcomes. Moreover, both these options are noncurative and cannot preserve the native heart function. Pulmonary artery banding (PAB) has been reinvented as a possible “regenerative surgery” to retrain the decompensated left ventricle in children with DCM. The rationale is to promote positive ventricular–ventricular interactions that result in recovery of left ventricular function in one out of two children, allowing transplantation delisting.
- pulmonary artery banding
- dilated cardiomyopathy
1. Introduction
2. Bedside: The Clinical Efficacy of PAB
After fifteen years from the first case report of the successful application of PAB in a 2-month-old baby with DCM [25][2], less than 20 centers worldwide have published their clinical results [28,29,30,31][3][4][5][6]. From these findings, what wresearchers have learned is that PAB has a concrete potential to improve LV function in 30% to 80% of treated patients, when RV function is preserved. Early mortality is low (10%), comparable, or even inferior to that associated with HTX or MCS in infants [1,32,33][1][7][8]. No directly PAB-related deaths have been described so far. The most common causes of exitus in PAB nonresponders are progressive deterioration of LV function or lethal complications following MCS implantation, which has been implanted as a rescue strategy [28,29][3][4]. This is in keeping with the natural history of DCM, where one-year survival after diagnosis drops to 80% in high-risk profiles [34][9], regardless of medical and surgical therapies. The international experience with PAB was consolidated in a multicenter study, for a total of 70 patients from 15 institutions worldwide [29][4]. In this cohort, PAB was adopted as part of different surgical strategies. In nine children, PAB served as a recovery strategy to wean patients with DCM following major open-heart procedures, with eight out of nine patients experiencing recovery. In the remaining 61 cases, PAB was performed as an isolated short open-chest approach. Among the latter, 8 patients underwent PAB as a bridge to HTX as an alternative to MCS, while in 53 children, PAB was intended as a true potential “regenerative surgery”. Focusing on the 61 patients who received PAB as the sole operation, outcomes are encouraging. Overall medium-term mortality is confirmed to be modest (8/61, 13%), and the rate of complete LV recovery is brilliant (34/61, 56%). In addition, 8 patients (13%) experienced a partial improvement in LV function, while 13 patients (21%) required an HTX, as a planned strategy or because of PAB nonresponders. Updates regarding these numbers are expected to further support the promising early results. More recently, a multicenter US study enrolling 14 children banded at a median of 5 months of age highlighted some differences with respect to the German experience [30][5]. Functional recovery was achieved in 29% of cases (4/14 patients), while 8 patients underwent HTX and 2 expired. The authors hypothesized that the more compromised preoperative status of patients in the US cohort, as well as the substantial differences in HF etiologies (myocarditis in 40% of patients in the German registry vs. 0% in the US study), contributed to the lower observed recovery rate. In fact, PAB was attempted as a rescue strategy to prevent committing to high-risk ventricular assist device implantation [30][5]. In this view, it is not surprising that a supposed regenerative strategy (PAB) that relies on the residual repair potential of the native heart and organism would be less beneficial when performed “too late”. Moreover, LV noncompaction, which wresearchers found as a possible contributing factor to PAB failure in ourresearchers' experience [31][6], was unusually frequent in the US cohort (5/14 patients, 39% vs. 8/61 cases, 13% in the world network report [29][4]), revealing the presence of a structural detrimental substrate, traditionally associated with relevant irreversible myocardial fibrosis [35][10], in a consistent quote of patients. Since 2015, wresearchers have embarked on a surgical protocol alternative to HTX to treat pediatric end-stage HF [31][6], including PAB. So far, wresearchers have treated with PAB a total of eight patients affected by DCM (four males, 50%) at a median age of 8 (5–10) months. In two cases (25%), the PAB strategy failed, and both patients required MCS with the Berlin Heart, followed by a successful HTX [31][6]. In one case (with a diagnosis of LV noncompaction and a known heterozygous mutation of the TPM1 gene and the ABCC9 gene), the PAB strategy was initially successful, allowing home discharge, but 3 months after PAB, the patient developed severe RV failure after acute pneumonia. Data regarding genetic testing in patients with DCM undergoing PAB are sparse in the scientific literature. The Giessen group never reported known mutations affecting their patients, nor gave specific recommendations on genetic testing before PAB. From the multicenter US cohort [30][5], only two patients were known to have genetic syndromes (including one patient with Leigh Syndrome and one patient with 12p duplication). In ouresearchers' center, genetic testing is always performed as part of the diagnostic protocol for patients with DCM. However, the decision to pursue the PAB strategy cannot benefit from such information, since the results of genetic tests require time, and patients usually necessitate a rapid treatment decision. The results of genetic testing are used to better guide the follow-up of patients and possibly predict the probabilities of recurrence of HF. As stated, only one patient in ourresearchers' cohort was found to possess a heterozygous mutation of the TPM1 gene (de novo) and the ABCC9 gene (parental). In four patients (50%) in ouresearchers' cohort, LV performance improved significantly, allowing transplantation delisting. In the remaining two patients (the most recent ones), LV function is still depressed, but clinical conditions are stable, and the patients are kept on an active transplantation waitlist (Figure 1). At a median follow-up time of 4.4 (1–5.4) years from PAB, all patients are alive and followed up regularly.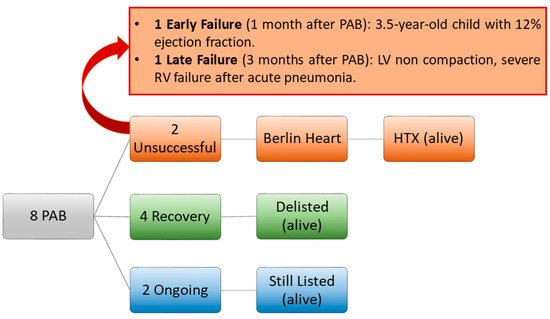
3. Bedside: Caveats from Clinical Experience
3.1. Age
The neonatal heart is demonstrated to possess a robust capacity for myocardial tissue regeneration, through the presence of highly active cardiac progenitor cells [36][11]. In fact, the cardiac tissue harvested from patients with congenital heart diseases can spontaneously generate mesenchymal stem cells in vitro [37][12], which is indicative of a preserved endogenous capacity of myocardial repair. Unfortunately, this repair potential is age-dependent, with a progressive and marked reduction beyond one year of age [37][12]. There is evidence that the regeneration capacity of the adult mammalian heart is very limited, due to the incapacity of cardiomyocytes to proliferate in adulthood. However, this is not true in neonates and infants, where a residual or reversible potential for proliferation has been reported [36][11]. Clinical experience with infants with complex congenital heart disease (i.e., anomalous left coronary artery from the pulmonary artery) has shown that after early surgical repair (i.e., coronary reimplantation), a full myocardial recovery with normalization of LV function is possible even after myocardial ischemia [38,39][13][14]. In addition, experience with rapid two-stage arterial switch in infants affected by transposition of the great arteries, who are too old to undergo a successful arterial switch operation, has shown the possibility of LV myocardial recovery after PAB, which has been able to induce an increase in cardiac mass to allow an uneventful late repair [40,41][15][16]. While cardiomyocytes show robust replicative activity during embryonic and fetal development, with subsequent waves of proliferation [42][17], replication stops after birth, never to resume again, at least not significantly. Further cardiac enlargement occurs by cell hypertrophy. The withdrawal of cardiomyocytes from the cell cycle after birth profoundly impacts on the capacity of the mammalian heart to undergo repair after damage: in the mouse, loss of myocardial tissue in the fetal or early neonatal life is healed through the generation of new contractile tissue [43][18], while fibrosis and scarring predominate later. The reason why cardiomyocyte proliferation stops irreversibly after birth seems to be linked to biochemical and mechanical events occurring after birth, such as the increase in oxygen tension and oxidative stress [44][19], the lack of maternal factors [45][20], the changes in hormonal stimulation [46][21], and, most notably, pressure overload [47][22], as the hydrodynamic modifications occurring in the newborn circulation result in a significant increase in cardiac afterload. All these observations may suggest that the beneficial effects of PAB in young patients are related to the specific induction of acute afterload increase, with consequent cardiomyocytes proliferation and cardiac regeneration. An ancient organ size control pathway (Hippo signaling) specifically inhibits cardiomyocyte proliferation in the adult heart [48,49][23][24]. Regulating the balance between cell differentiation, proliferation, and apoptosis, the Hippo signaling pathway also governs cardiac fibroblasts’ function and, subsequently, heart fibrosis [50][25], together with the endothelial response to oxidative stress, inflammation, and angiogenesis [51][26]. For these reasons, the inactivation of the Hippo pathway has been proposed as part of the putative strategies to promote myocardial regeneration after an injury [52][27]. If proven, PAB might act on these molecular pathways, modifying intracardiac pressure parameters that are detected by the cardiac mechano-sensing apparatus, which can regulate the Hippo signaling [53,54][28][29]. In fact, the dystrophin–glycoprotein complex, which links the cytoskeleton of myocardial fibers to the extracellular matrix, can regulate the Hippo pathway by binding its principal effector YAP (Yes-associated protein), modulating cardiomyocyte proliferation in mice [53][28]. This protein is regulated by the mechanical stress and the extracellular matrix stiffness, which are obliviously affected by the pressure overload imposed by PAB on the RV chamber. In ouresearchers' experience, a possible cause of PAB failure is the age of patients [31][6]. Schranz et al. suggested a 6-year-old age limit for PAB in DCM [55][30]. However, wresearchers believe that a lower threshold should be applied. Relying on the repair ability of the young heart and the antiregenerative pathways that aging activates in the myocardium, weresearchers now consider PAB as a surgical option only in children under 1 year of age (Figure 2) [31][6]. A similar philosophy has emerged in the setting of retraining of the morphological LV in congenitally corrected transposition of the great arteries, where the maximal age threshold for patients to be considered for PAB has been lowered across the years. In 2005, Winlaw et al. observed that patients >16 years at PAB were unlikely to achieve a definitive anatomical repair [56][31]. Eight years later, Myers et al. described an increased risk of LV dysfunction when PAB was performed after 2 years of age [57][32]. In the most recent series, the median age at PAB is even below 1 year [58][33], reflecting a trend toward the anticipation of PAB in early infancy.
3.2. Associated Cardiac Defects
In nine patients from the worldwide multicenter cohort [29][4], PAB was performed in association with other major surgical procedures (mitral valve repair/replacement, correction of an anomalous left coronary artery arising from the pulmonary artery, repair of partial anomalous pulmonary venous return, etc.). However, wresearchers consider major associated cardiac defects as relative contraindications to PAB [31][6], as suggested by the selection criteria proposed by Schranz et al. [55][30]. One of the most important advantages of PAB is the minimal surgical invasiveness, requiring neither cardioplegic arrest of the heart nor cardiopulmonary bypass. As described in adult cardiac diseases, the more profound the LV dysfunction is, the less the cardioplegic arrest of the heart is tolerated [59][34]. Due to the higher density of Thebesian and arterio-luminal vessels that may contribute to a more rapid washout of the cardioplegic solution [60[35][36],61], the RV is historically known to be more susceptible to ischemic injury during cardiopulmonary bypass [62,63,64,65][37][38][39][40]. On a molecular basis, in patients with HF, the ischemic/reperfusion injury associated with the cardioplegic arrest of the heart results in an exasperated inflammatory response and myocardial cytokine release when compared to nonfailing hearts [66][41]. Thus, the cardioplegic arrest needed to perform the surgical repair of associated intracardiac defects could impair the hypothesized immunomodulatory effects of PAB in pediatric DCM. Consequently, the abrupt overwork induced by PAB during the delicate phases of coronary reperfusion may result in acute RV failure rather than a hypertrophic positive adaptation. For this reason, wresearchers perform PAB mostly as an isolated procedure at ourresearchers' institution, to minimize myocardial injury and optimize the efficacy of PAB (Figure 2). In the case of substantial structural defects to be addressed, alternative strategies are considered [31][6].3.3. MCS Backup
According to the worldwide network report by Schranz et al. [29][4], PAB in DCM can serve different purposes: 1. as a recovery strategy to wean patients with DCM following major open-heart procedures; 2. as a bridge to a planned HTX to avoid MCS support; 3. as a bridge to recovery. Nonresponders to PAB range from 30% to 60% of treated patients, in the European and US experiences, respectively [28,30,31][3][5][6]. Lower recovery rates may reflect a more compromised preoperative status, when the detrimental cascade of HF is less likely to be reverted [30][5]. Moreover, the immediate postoperative days represent a critical period for achieving adequate circulatory stabilization, and acute insults may dramatically result in biventricular failure [29][4]. In this setting, a prompt instauration of MCS is the only chance to sustain the patient until a compatible donor is available. In ouresearchers' experience, two children in whom PAB was unsuccessful both required temporary circulatory support with the Berlin Heart, before undergoing an uneventful HTX [31][6]. Similarly, seven out of eight patients who manifested an early or late deterioration after PAB in the US cohort were placed on ventricular assist device support until a compatible donor was available [30][5]. Moreover, in ouresearchers' experience, a temporary MCS has also been helpful in two more compromised patients who experienced low-cardiac-output syndrome after PAB. They were assisted on venous–arterial ECMO for a few days until a successful and uneventful weaning off. For this reason, wresearchers strongly recommend that PAB be considered as a surgical option exclusively in patients who are eligible for long-term MCS. Complete patient assessment for MCS, family counseling, and notification to manufacturers must be integral parts of the preoperative planning of PAB (Figure 2). Since it is well-recognized that surgical volume is a determinant of MCS outcomes [67][42], a solid background in pediatric MCS and ECMO support is mandatory before including PAB in the institutional surgical protocol for end-stage HF. With more patients being treated with PAB, wresearchers expect further refinements of selection criteria, possibly stratifying candidates based on the risk of PAB failure, and guiding a more targeted planning of an MCS backup.References
- Rossano, J.W.; Cherikh, W.S.; Chambers, D.C.; Goldfarb, S.; Hayes, D.J.; Khush, K.K.; Kucheryavaya, A.Y.; Toll, A.E.; Levvey, B.J.; Meiser, B.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric heart transplantation report-2018; Focus theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1184–1195.
- Schranz, D.; Veldman, A.; Bartram, U.; Michel-Behnke, I.; Bauer, J.; Akintürk, H. Pulmonary artery banding for idiopathic dilative cardiomyopathy: A novel therapeutic strategy using an old surgical procedure. J. Thorac. Cardiovasc. Surg. 2007, 134, 796–797.
- Schranz, D.; Rupp, S.; Müller, M.; Schmidt, D.; Bauer, A.; Valeske, K.; Michel-Behnke, I.; Jux, C.; Apitz, C.; Thul, J.; et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: A novel therapeutic strategy before heart transplantation. J. Heart Lung Transplant. 2013, 32, 475–481.
- Schranz, D.; Akintuerk, H.; Bailey, L. Pulmonary Artery Banding for Functional Regeneration of End-Stage Dilated Cardiomyopathy in Young Children: World Network Report. Circulation 2018, 137, 1410–1412.
- Spigel, Z.A.; Razzouk, A.; Nigro, J.J.; Karamlou, T.B.; Kavarana, M.N.; Roeser, M.E.; Adachi, I. Pulmonary Artery Banding for Children with Dilated Cardiomyopathy: US Experience. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2020, 23, 69–76.
- Ponzoni, M.; Frigo, A.C.; Castaldi, B.; Cerutti, A.; Di Salvo, G.; Vida, V.L.; Padalino, M.A. Surgical strategies for the management of end-stage heart failure in infants and children: A 15-year experience with a patient-tailored approach. Artif. Organs 2021, 45, 1543–1553.
- Morales, D.L.S.; Adachi, I.; Peng, D.M.; Sinha, P.; Lorts, A.; Fields, K.; Conway, J.; Louis, J.D.S.; Cantor, R.; Koehl, D.; et al. Fourth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report. Ann. Thorac. Surg. 2020, 110, 1819–1831.
- Rossano, J.W.; Singh, T.P.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.J.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report—2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1028–1041.
- Rossano, J.W.; Kantor, P.F.; Shaddy, R.E.; Shi, L.; Wilkinson, J.D.; Jefferies, J.L.; Czachor, J.D.; Razoky, H.; Wirtz, H.S.; Depre, C.; et al. Elevated Heart Rate and Survival in Children with Dilated Cardiomyopathy: A Multicenter Study from the Pediatric Cardiomyopathy Registry. J. Am. Heart Assoc. 2020, 9, e015916.
- Nucifora, G.; Aquaro, G.D.; Pingitore, A.; Masci, P.G.; Lombardi, M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur. J. Heart Fail. 2011, 13, 170–176.
- Sharma, S.; Mishra, R.; Bigham, G.E.; Wehman, B.; Khan, M.M.; Xu, H.; Saha, P.; Goo, Y.A.; Datla, S.R.; Chen, L.; et al. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circ. Res. 2017, 120, 816–834.
- Traister, A.; Patel, R.; Huang, A.; Patel, S.; Plakhotnik, J.; Lee, J.E.; Medina, M.G.; Welsh, C.; Ruparel, P.; Zhang, L.; et al. Cardiac regenerative capacity is age- and disease-dependent in childhood heart disease. PLoS ONE 2018, 13, e0200342.
- Latus, H.; Gummel, K.; Rupp, S.; Mueller, M.; Jux, C.; Kerst, G.; Akintuerk, H.; Bauer, J.; Schranz, D.; Apitz, C. Cardiovascular magnetic resonance assessment of ventricular function and myocardial scarring before and early after repair of anomalous left coronary artery from the pulmonary artery. J. Cardiovasc. Magn. Reson. 2014, 16, 3.
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart after Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221.
- Duan, Y.; Sun, Y.; Dong, S.; Du, C.; Yan, J. Two-Stage Arterial Switch for Transposition of the Great Vessels in Older Children. Ann. Thorac. Surg. 2022, 114, 193–200.
- Boutin, C.; Jonas, R.A.; Sanders, S.P.; Wernovsky, G.; Mone, S.M.; Colan, S.D. Rapid two-stage arterial switch operation. Acquisition of left ventricular mass after pulmonary artery banding in infants with transposition of the great arteries. Circulation 1994, 90, 1304–1309.
- Sedmera, D.; Reckova, M.; DeAlmeida, A.; Coppen, S.R.; Kubalak, S.W.; Gourdie, R.G.; Thompson, R.P. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 274, 773–777.
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080.
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579.
- Zacchigna, S.; Martinelli, V.; Moimas, S.; Colliva, A.; Anzini, M.; Nordio, A.; Costa, A.; Rehman, M.; Vodret, S.; Pierro, C.; et al. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat. Commun. 2018, 9, 2432.
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188.
- Canseco, D.C.; Kimura, W.; Garg, S.; Mukherjee, S.; Bhattacharya, S.; Abdisalaam, S.; Das, S.; Asaithamby, A.; Mammen, P.P.; Sadek, H.A. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 2015, 65, 892–900.
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461.
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo signaling impedes adult heart regeneration. Development 2013, 140, 4683–4690.
- Tsai, C.-R.; Martin, J.F. Hippo signaling in cardiac fibroblasts during development, tissue repair, and fibrosis. Curr. Top. Dev. Biol. 2022, 149, 91–121.
- Zhang, W.; Li, Q.-Q.; Gao, H.-Y.; Wang, Y.-C.; Cheng, M.; Wang, Y.-X. The regulation of yes-associated protein/transcriptional coactivator with PDZ-binding motif and their roles in vascular endothelium. Front. Cardiovasc. Med. 2022, 9, 925254.
- Valizadeh, A.; Asghari, S.; Mansouri, P.; Alemi, F.; Majidinia, M.; Mahmoodpoor, A.; Yousefi, B. The Roles of Signaling Pathways in Cardiac Regeneration. Curr. Med. Chem. 2022, 29, 2142–2166.
- Morikawa, Y.; Heallen, T.; Leach, J.; Xiao, Y.; Martin, J.F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 2017, 547, 227–231.
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770.
- Schranz, D.; Recla, S.; Malcic, I.; Kerst, G.; Mini, N.; Akintuerk, H. Pulmonary artery banding in dilative cardiomyopathy of young children: Review and protocol based on the current knowledge. Transl. Pediatr. 2019, 8, 151–160.
- Winlaw, D.S.; McGuirk, S.P.; Balmer, C.; Langley, S.M.; Griselli, M.; Stümper, O.; De Giovanni, J.V.; Wright, J.G.; Thorne, S.; Barron, D.J.; et al. Intention-to-treat analysis of pulmonary artery banding in conditions with a morphological right ventricle in the systemic circulation with a view to anatomic biventricular repair. Circulation 2005, 111, 405–411.
- Myers, P.O.; del Nido, P.J.; Geva, T.; Bautista-Hernandez, V.; Chen, P.; Mayer, J.E.J.; Emani, S.M. Impact of age and duration of banding on left ventricular preparation before anatomic repair for congenitally corrected transposition of the great arteries. Ann. Thorac. Surg. 2013, 96, 603–610.
- Ibrahimiye, A.N.; Mainwaring, R.D.; Patrick, W.L.; Downey, L.; Yarlagadda, V.; Hanley, F.L. Left Ventricular Retraining and Double Switch in Patients with Congenitally Corrected Transposition of the Great Arteries. World J. Pediatr. Congenit. Heart Surg. 2017, 8, 203–209.
- Trescher, K.; Bauer, M.; Dietl, W.; Hallström, S.; Wick, N.; Wolfsberger, M.; Ullrich, R.; Jurgens, G.; Wolner, E.; Podesser, B.K. Improved myocardial protection in the failing heart by selective endothelin-A receptor blockade. J. Thorac. Cardiovasc. Surg. 2009, 137, 1005–1011.e1.
- Ansari, A. Anatomy and clinical significance of ventricular Thebesian veins. Clin. Anat. 2001, 14, 102–110.
- Crystal, G.J.; Pagel, P.S. Right Ventricular Perfusion: Physiology and Clinical Implications. Anesthesiology 2018, 128, 202–218.
- Mankad, P.S.; Yacoub, M.H. Systolic and diastolic function of both ventricles after prolonged cardioplegic arrest. Ann. Thorac. Surg. 1993, 55, 933–939.
- Abdul-Ghani, S.; Skeffington, K.L.; Kim, M.; Moscarelli, M.; Lewis, P.A.; Heesom, K.; Fiorentino, F.; Emanueli, C.; Reeves, B.C.; Punjabi, P.P.; et al. Effect of cardioplegic arrest and reperfusion on left and right ventricular proteome/phosphoproteome in patients undergoing surgery for coronary or aortic valve disease. Int. J. Mol. Med. 2022, 49, 77.
- Donauer, M.; Schneider, J.; Jander, N.; Beyersdorf, F.; Keyl, C. Perioperative Changes of Right Ventricular Function in Cardiac Surgical Patients Assessed by Myocardial Deformation Analysis and 3-Dimensional Echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 708–718.
- Murphy, C.O.; Pan-Chih Gott, J.P.; Guyton, R.A. Microvascular reactivity after crystalloid, cold blood, and warm blood cardioplegic arrest. Ann. Thorac. Surg. 1995, 60, 1021–1027.
- Kortekaas, K.A.; Lindeman, J.H.; Versteegh, M.I.; van Beelen, E.; Kleemann, R.; Klautz, R.J. Heart failure determines the myocardial inflammatory response to injury. Eur. J. Heart Fail. 2013, 15, 400–407.
- Morales, D.L.S.; Rossano, J.W.; VanderPluym, C.; Lorts, A.; Cantor, R.; St. Louis, J.D.; Koeh, D.; Sutcliffe, D.L.; Adachi, I.; Kirklin, J.K.; et al. Third Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report: Preimplant Characteristics and Outcomes. Ann. Thorac. Surg. 2019, 107, 993–1004.
