Point-of-care testing (POCT) technology has exhibited an outstanding capability for the detection of several disease biomarkers owing to the fact that such techniques are fast, easy to perform, efficient, and low cost. The lateral flow immunoassay (LFIA) is one such strategy for POCT. LFIA is a well-established platform and a potent assay for fast and inexpensive testing, as this technology is instrumentation independent and allows the visualisation of test results by the naked eye. In order to enhance the sensitivity and specificity of the LFIAs, as well as to allow the quantitation of results, fluorescence immunochromatographic assays have been developed by utilising fluorescent reporters [16,17,18,19,20]. Fluorescence immunochromatographic assays have advantages over conventional approaches in regard to sensitivity as it produces a higher intensity band on the test and control lines [21,22]. One such promising fluorescent reporter is quantum dots (QDs). QDs are tiny semiconducting nanocrystals with diameters ranging from 2 to 10 nanometers. QDs have unique electronic characteristics that are intermediate between those of bulk semiconductors and discrete molecules, which is due in part to their high surface-to-volume ratios. The most visible result is fluorescence, in which the nanocrystals emit distinct colours determined by particle size. Hence, the present review focuses on a quantum dot-based lateral flow immunoassay (QD-LFIA).
- quantum dot
- lateral flow immunoassay
- infectious diseases
1. Lateral Flow Immunoassay

1.1. Sandwich Immunoassay
Sandwich LFIAs have been widely used for the detection of various disease biomarkers as well as small molecules, such as vitamin D [6][28]. Three different antibodies are usually used in this format [7][29]. (i) Conjugate antibodies recognise one of the target analyte’s epitopes, and they are immobilised on a conjugation pad linked to the reporter particles. When the sample is added, the conjugate antibody rehydrates and migrates to the test and control lines via capillary force. (ii) Capture antibodies are immobilised on the nitrocellulose membrane at the test line and are specific to another epitope of the target analyte. (iii) Species-specific anti-immunoglobulin antibodies that are immobilised on the control line membrane interact with the reaction antibody. When the specimen is applied to the sample pad, the target analyte binds with the conjugate antibody on the conjugate pad (analyte–Ab complex) and it is directed to the nitrocellulose membrane. This complex reacts with detection antibodies on the test line, resulting in the sandwich shown in Figure 2. On the control line, excess reaction antibody reacts with species-specific anti-immunoglobulin antibodies. Two red lines will appear at the test and control lines, indicating the presence of the target analyte [8][30]. In the absence of a target analyte, the reaction antibody only reacts with the species-specific anti-immunoglobulin antibody on the control line. At the control line, only one red line will appear. The presence of a control line indicates that the flow is completed and the test is valid. There are various strategies have been employed at the control line. The most popular approach is by utilising species-specific anti-IgG (Figure 2). Alternatively, researchers have also used an independent antibody–antigen complex such as biotinylated antibodies or oligonucleotides and bovine serum albumin-biotin conjugate [9][10][11][31,32,33].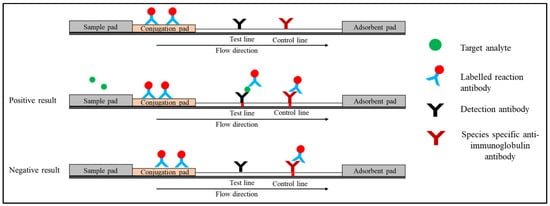
1.2. Competitive Immunoassay (or Inhibition Immunoassay)
The competitive immunoassay format, also known as the inhibition immunoassay, can be conducted in two types of arrangements. In the first arrangement (Figure 3), the labelled analyte is attached to the conjugation pad. In the absence of the target analyte in the specimen, the labelled analyte moves through the strip bind detection antibody on the test line and the secondary antibody on the control line. The red colour will appear on both the test and control lines. In the presence of the target analyte, the unlabelled analyte in the specimen competes with the labelled analyte and binds to the test line. In the control line, the labelled analyte binds to the secondary antibody. Only a red line is formed on the control line [12][34].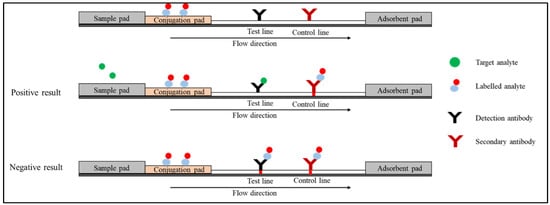
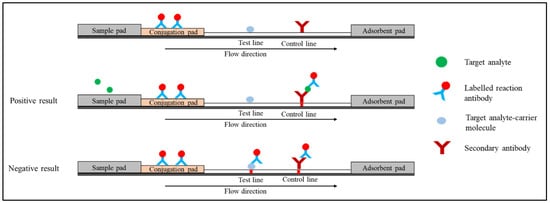
2. Quantum Dot-Based Lateral Flow Immunoassay
Quantum dots (QDs) are the most auspicious fluorescent reporters due to their unique properties, which include high stability, high extinction coefficients, high quantum yields, and a long fluorescence lifespan [14][15][38,39]. These characteristics make QDs excellent reporters that can be functionalised with conjugate antibodies for the development of highly sensitive LFIAs. A schematic illustration of the QDs functionalised with conjugate antibodies in sandwich LFIAs is illustrated in Figure 5. The use of QDs as a reporter in sandwich LFIAs requires two antibodies wherein both the conjugate and capture antibodies are specific to the target of interest. Conjugate antibodies labelled with QDs are immobilised on the conjugate pad to enable measurable fluorescence detection. Meanwhile, on a nitrocellulose membrane, capture antibodies are immobilised to capture the target of interest, forming the QD-labelled antibody–target–antibody complex. This complex produces a bright fluorescent band in response to ultraviolet excitation. Similarly, QDs can also be used in competitive LFIAs format by functionalising the nanoparticles with reaction antibodies or analytes on the conjugate pad.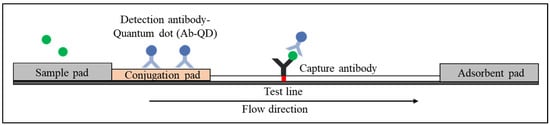
2.1. Types of Quantum Dots
2.2. Strategies for Conjugating Quantum Dot with Antibodies
The process of conjugating antibodies to the surface of QDs can be performed using several methods. Passive adsorption is the established technique for conjugating antibodies to the surface of QDs and is still widely used. Utilising the interactions (forces) between molecules and surfaces at a certain pH (e.g., van der Waals and ionic forces), antibodies can be directed to spontaneously bind to QDs to form a conjugate. The antibody is normally added in excess to certify that the entire surface of the QDs is covered. After the conjugation is finished, any antibody that is still free in the solution is removed using centrifugation or filtration. Covalent binding is the most commonly used method for conjugating antibodies to the QDs surface. Covalent binding of QD to conjugate antibodies offers several advantages. Fewer numbers of antibodies are needed to increase the sensitivity, thus lowering the overall cost. Furthermore, covalent conjugates exhibit high stability, allowing their use in difficult sample matrices and high-salt buffering solutions. Additionally, conjugates are easily prepared without the need for extensive salt or pH optimisations, thus, meaning antibody screening experiments can be performed faster. One common strategy for employing covalent binding is by functionalising the QD surfaces with carboxyl (carboxylic acid). However, obtaining QDs functionalised with carboxyl groups could be tricky. There are several methods to synthesise carboxyl-functionalised QDs have been previously reported. Mansur et al. [16][46] used acid-functionalised poly (vinyl alcohol) (PVA–COOH) polymer as a capping ligand to synthesise CdSe nanoparticles. The synthesis method was performed using the colloidal chemistry technique via the aqueous route at room temperature. Alternatively, Chen et al. [17][47] dissolved QDs in deionised water using an ultrasonic bath. Then, HCl solution was added to the solution and mechanically stirred to replace Na+ ions with H+, producing carboxyl-functionalised QD. Once the QDs functionalised with carboxyl groups, the carboxylic groups on QD surfaces can be linked to a primary amine in lysine residues of antibody using 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysulfosuccinimide (NHS) reagents to form amide bonds [18][19][48,49]. Another approach is via reductive amination, by oxidising the oligosaccharides on the Fc region of the antibody followed by coupling to the amine- or hydrazide-functionalised QDs [20][50].2.3. Performance of Quantum Dot-Based Lateral Flow Immunoassay
1.3. Performance of Quantum Dot-Based Lateral Flow Immunoassay
Syphilis is a sexually transmitted infection (STI) that causes serious health problems worldwide. In 2010, Yang et al. [21][56] developed a QD-LFIA to be used for the screening of syphilis. The QD-LFIA was designed to detect anti-TP47 polyclonal antibodies by visualising the emission of CdTe under a portable ultraviolet lamp. Thioglycolic acid (TGA) was used to link the QDs with Staphylococcal Protein A (SPA). In the presence of anti-TP47 polyclonal antibodies, the QD-labelled SPA will form a complex with the antibodies. Then, the anti-TP47 antibodies will bind to the TP47 antigen immobilised on the test line, producing a signal that can be visualised under UV light. The assay is suitable for rapid indirect screening of syphilis as the turnaround time is only 10 min. With regard to the limit of detection, the QD-LFIA could detect as low as 2 ng/mL, which was tenfold higher than that of the AuNPs–based method.
Tuberculosis, an infectious disease caused by the Mycobacterium tuberculosis, is a global public health problem and among the top 10 leading causes of death worldwide, particularly in low- and middle-income countries [22][59]. In an effort to prevent the spread of the disease, a QD-LFIA device based on a double-antibody sandwich format labelled with core–shell quantum dots (CdSe/ZnS) coupled with streptavidin was developed for the detection of M. tuberculosis fprA proteins [23][51]. The Itstudy reported that the device was able to detect fprA proteins in liquid samples at the lowest dilution of 12.5 pg/µL. The sensitivity of LFIA improved as compared to other immunochromatographic tests following the use of QDs labelled antibodies.
