A complex balanced equilibrium of the bacterial ecosystems exists in the oral cavity that can be altered by tobacco smoking, psychological stressors, bad dietary habit, and chronic periodontitis. Oral dysbiosis can promote the onset and progression of oral squamous cell carcinoma (OSCC) through the release of toxins and bacterial metabolites, stimulating local and systemic inflammation, and altering the host immune response. During the process of carcinogenesis, the composition of the bacterial community changes qualitatively and quantitatively. Bacterial profiles are characterized by targeted sequencing of the 16S rRNA gene in tissue and saliva samples in patients with OSCC. The human microbiota may determine the response to cancer therapy through different mechanisms, and thus may positively or negatively influence the outcome.
1. Oral Microenvironment in Healthy Condition
The interspecies and host-microbial interactions can be included in the holistic framework of holobiont, which accounts for the host and its obligate or facultative symbionts, whose dynamic interconnectedness entails emergent interactions and outcomes
[1]. Despite the fact that the notorious 10:1 ratio of bacterial/human cells has been revised to the estimate of about 1.3:1 in a “reference man”, with about 38 × 10
12 total bacteria in the human body
[2], the biological role of the microbiota has not scaled down. The number of bacteria in the mouth is less than 1% of the colon bacteria number; however, bacterial concentrations in the saliva and dental plaque are high (about 109 and 1011, bacteria per mL content, respectively)
[2]. Comprehensive information about the bacterial species present in the oral cavity is publicly available (Expanded Human Oral Microbiome Database (EHOMD), n.d.). Multi-omics approaches are currently available to study the complexity of polymicrobial environments, interspecies interactions and the mechanisms underlying these relationships
[3].
Changes in microbial biomass in the oral cavity depend on the interaction between different microbial species in the biofilm. The oral biofilm matures through interactions between early colonizing microorganisms with later colonizers through several mechanisms, including coagulation, metabolic exchange, small-molecule signal-mediated communication, and exchange of genetic material. The numerous bacteria in the oral cavity are not uniformly distributed over all surfaces but proliferate differently in ecological niches depending on their metabolism in a healthy oral cavity
[4].
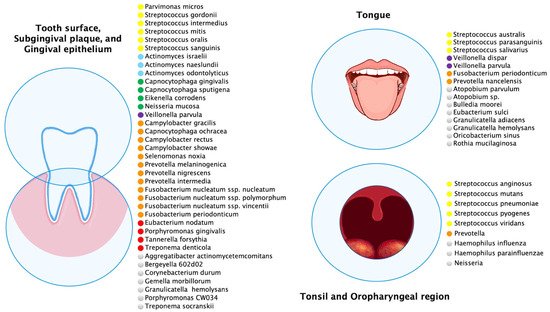
Multiple oral anatomical sites influence the ecology of these habitats and create microbial environmental differences (
Figure 1). The most abundant species of buccal epithelium were
Streptococcus,
Gemella,
Eubacterium,
Selenomonas,
Veillonella,
Actinomyces,
Atopobium,
Rothia,
Neisseria,
Eikenella,
Campylobacter,
Porphyromonas,
Prevotella,
Granulicatella,
Capnocytophaga,
Fusobacterium,
Leptotrichia,
Streptococcus mitis,
Granulicatella adiacens, often considered an opportunistic pathogen, was detected from bacteremia/septicemia in patients with infective endocarditis/atheroma
[5] but also at buccal sites in the healthy oral cavity.
On the tongue dorsum between keratinized papillae filiformis there were species of
Streptococcus mitis,
Streptococcus australis,
Streptococcus parasanguinis,
Streptococcus salivarius,
Streptococcus sp. clone FP015, and
Streptococcus sp. clone FN051,
Granulicatella adiacens, and
Veillonella spp. Anatomical differences of the dorsum of the tongue from the lateral margin influenced the bacterial profiles above. The lateral margin of the tongue has a smooth nonkeratinized surface, and the predominant bacteria were
Streptococcus mitis,
Streptococcus mitis bv. 2,
Streptococcus sp. clone DP009,
Streptococcus sp. clone FN051,
Streptococcus australis,
Granulicatella adiacens,
Gemella haemolysans, and
Veillonella spp.
[6][7][6,7]. The tongue species most associated with health were
Streptococcus salivarius,
Rothia mucilaginosa (
Stomatococcus mucilaginosus), and an uncharacterized, cultivable species of
Eubacterium (strain FTB41)
[8].
Likewise, the main bacterial species were
Streptococcus mitis,
Streptococcus mitis biovar 2,
Streptococcus sp. clone FN051,
Streptococcus infantis,
Granulicatella elegans,
G. hemolysans, and
Neisseria subflava on the hard palate. Streptococcus mitis was the most commonly found species in essentially all healthy sites and subjects. However, both
S. mitis and
Streptococcus oralis have been associated with bacterial endocarditis in patients with prosthetic valves
[9][10][9,10]. In addition to the distinctive bacteria of a healthy oral cavity there were bacteria associated with periodontal disease, such as
Porphyromonas gingivalis,
Tannerella forsythia, and
Treponema denticola [11].
Figure 1. Predominant microbial communities within different ecological niches of the oral and oropharyngeal region. This figure was created using data sources from Aas et al.
[6], Dewhirst et al.
[12], Diaz et al.
[13], Hall et al.
[14], and Tamashiro et al.
[15]. The species were color-coded according to microbial complexes described by Haffajee et al.
[16].
2. Microbiota and Inflammation in The Oral Cavity
Oral health firstly depends on a correct balance between the various microbial populations that constitute the oral microbiota, when this balance is lost, a condition of dysbiosis is established. A condition of dysbiosis occurs, in fact, as a consequence of the disproportionate proliferation of some types of pathogenic microorganisms and it can have several triggers such as antimicrobial agents, age, hormonal changes, poor oral hygiene, smoking, or it may be favored by other body pathologies. Dysbiosis can lead to the generation of numerous oral diseases, including carious pathology, periodontal disease, and gingivitis
[17].
Both periodontitis and gingivitis are characterized by inflammation, but periodontitis is a more serious condition than gingivitis. In the latter, the inflammation is limited to the soft tissues surrounding the teeth while periodontitis is a chronic disease which results in the destruction of teeth supporting structures and it is recognized as the leading cause of tooth loss in the adult population of industrialized countries.
Periodontal disease is defined as a chronic inflammatory disease and the bacteria responsible for this disease can persist, resistant to immune reactions, especially if the disease is not treated. Inflammation is part of the body’s first line of defense against invasive pathogens. A correct inflammatory response guarantees the correct resolution of the inflammation, but when the inflammatory reactions are inadequate and persistent over time there can be serious damage both locally and systemically.
Immune responses are subjected to a complex regulation that is the result of interactions between different types of immunocompetent cells, which communicate to each other through cytokines which have the function of regulating, suppressing, or amplifying the mechanisms of inflammation. Inevitably, periodontitis leads to an increase of proinflammatory cytokines such as interleukin- (IL-) 1α, IL-1 β, tumor necrosis factor-alpha (TNFα), and IL-6. IL-8 is a chemokine, important for the recruitment of defense cells in areas where their presence is necessary. IL-17 also seems to be involved in the pathogenesis of periodontal disease. It is a proinflammatory cytokine that stimulates the production of other mediators, such as IL-6 and matrix metalloproteinases (MMPs). The prostaglandins E2 (PGE2), which are vasoactive amines derived from arachidonic acid, are also involved in the inflammatory process. They act on fibroblasts and osteoclasts by stimulating the production of MMPs. The helper T cells regulate both humoral and cell-mediated immune responses. The humoral immune response is promoted by Th2 cells, which produce IL-4, IL-5, IL-10, and IL-13. Th1 lymphocytes release IL-2 and interferon-γ (INF-γ) which increases cell-mediated responses. The relationship between inflammation and cancer is not new. Recently, a very high number of studies have discussed this issue. Many malignancies arise from areas of infection and inflammation, simply as part of the normal host response. Indeed, there is a growing body of evidence that shows that many malignancies are initiated by infections; upwards of 15% of malignancies worldwide can be attributed to infections, a global total of 1.2 million cases per year
[18][19][18,19]. P53 mutations are seen at frequencies similar to those in tumors in chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease
[18].
Undoubtedly, inflammation plays an important role in carcinogenesis and interestingly, several oral bacteria have been shown to activate inflammatory pathways associated with several stages of cellular transformation. Pathogenic bacteria can promote malignant tumors, and the tumor microenvironment itself can selectively stimulate the growth of bacteria
[20]. The robust relationship between bacteria, chronic inflammation, and tumors is established: the functionally proinflammatory bacteriome in the body of the tumor is associated with oral cancer
[21].
Antiapoptotic pathways—such as the JAK/STAT and phosphatidylinositol 3-kinase (PI3K)/Akt—can be activated by infected gingival epithelial cells (GECs).
Porphyromonas gingivalis, a bacterium involved in periodontitis, can be internalized by GECs. Both pathways are also related to inflammation. Some cytokines such as IL-6, TNF-α, or IFN-γ function through the JAK/STAT pathway; additionally, the JAK/STAT pathway activates NF-κB and stimulates TNF-α production. The PI3K/Akt pathway, on the other hand, is involved in the increase of Toll-Like receptor-4 (TLR4) mRNA, in response to bacterial lipopolysaccharide (LPS). Finally, phosphorylation of Akt and its consequent activation induces NF-κB, which increases the transcription of antiapoptotic genes
[19]. Coinfection studies using
Fusobacterium nucleatum and
Porphyromonas gingivalis show that they induce a synergic virulence response with a stronger inflammatory response triggered by elevated levels of TNF-α, NF-κB, and interleukin IL-1β, as well as higher levels of attachment and invasion into host cells
[19].
Another interesting role is played by MMPs, which are a family of zinc-dependent endopeptidases with more than 20 different members. Matrix metalloproteinases play a crucial role in extracellular matrix and basement membrane degradation. This property favors periodontal tissue destruction, as well as cancer progression and metastasis, by causing tissue dissolution that enables tumor invasion
[22]. Different studies have demonstrated higher concentrations of MMP-9 in patients with chronic periodontitis than in controls. MMP-9 is a widely investigated MMP, it is involved in cancer cell invasion, tumor metastasis, angiogenesis, and endothelial–mesenchymal-transition (EMT). It is also involved in mediating tumor microenvironment and modulating tumor-associated inflammation via cytokines and their receptors. The overexpression of MMP-9 has often been observed in different malignant tumors
[23][24][25][23,24,25].
Another important role in the link between bacteria-inflammation and cancerogenesis is the production of free radical species. Different studies have shown that periodontitis is related to excessive reactive oxygen species (ROS) production or elevated oxidative damage
[26].
Leukocytes and other phagocytic cells can induce DNA damage or interfere with DNA repair mechanisms, through their generation of reactive oxygen and nitrogen species that are produced normally by these cells to fight infection. These species react to the formation of peroxynitrite, a mutagenic agent. The generated products, in turn, demonstrate a strong affinity for more inflammatory cells, perpetuating the vicious cycle.
Many proinflammatory cytokines (such as IFN-γ, IL1β and other cytokines) facilitate induction of the inducible isoform of nitric oxide (NO) synthase (NOS), thus they could mediate excessive production of NO. NO facilitates vascular permeability, which accelerates nutritional supply to the tumor tissue and hence sustains the rapid tumor growth. This evidence suggests that inflammatory responses induced by various pathogens would accelerate mutagenesis as well as tissue damage
[18][27][18,27]. Bacteria and host might interact according to the plaque ecological hypothesis
[28]. Accumulation of supragingival and subgingival biofilms leads to inflammation, promoting alteration in physiological microbial composition. Such accumulation also increases the competitiveness of the oral pathogen at the expense of oral health-associated species through increased up-regulation of virulence factor expression. This results in a positive feedback loop.
The published studies on periodontitis and cancer mostly point to a positive association. In this association, inflammation has an essential role but more in-depth studies are needed. Sustained cell proliferation in an environment rich in inflammatory cells, activated stroma, and DNA-damage-promoting agents, certainly potentiates and/or promotes neoplastic risk. It is unclear whether the inflammatory mediators are critical for the development and growth of tumors or whether they constitute a permissive environment for the progression of malignancies. Moreover, a large number of studies show how the treatment of periodontitis substantially decreases markers of inflammation, but more investigation is also needed to assess how improved periodontal disease prevention and management strategies may impact cancer risk
[18][22][18,22].