2. Neurobiological Links between TBI and Neurological Dysfunctions
TBI is defined as an alteration in brain structure, or other evidence of brain pathology, caused by an external force
[10][11][10,11]. From the aspect of distribution of structural damage,
rwe
searchers can classify the injury to focal or diffuse
[12]. Focal brain injury is caused by the outside forces acting on the skull and resulting in compression of the tissue underneath the cranium at the site of the impact or the tissue opposite to the impact
[13]. The location of the impact to the skull determines the cerebral pathology and neurological deficits. By definition, diffuse brain injury is more scattered, and is not linked to a specific focus of destructive tissue damage
[14], including widely distributed damage to axons, diffuse vascular injury, hypoxic–ischemic injury, and brain swelling. Regardless of focal or diffuse injury, the characteristic of TBI involves the mechanisms of primary and secondary brain injury
[15]. Primary brain injury occurs at the exact moment of insult and results in the disruption of cell plasma membrane
[16]. Secondary brain injury occurs after primary brain injury and involves the participation of complicated mechanisms, including excitatory toxicity, mitochondrial dysfunction, oxidative stress, lipid peroxidation, neuroinflammation, and axonal degeneration, and, finally, it induces diverse forms of programmed cell death, such as necroptosis, autophagy, apoptosis, pyroptosis, and ferroptosis
[17][18][19][20][21][17,18,19,20,21]. TBI induces an increased mitochondrial membrane permeability. Mitochondria trigger a variety of apoptotic signaling pathways via interactions among the bcl-2 family proteins in order to release pro-apoptotic proteins from the intermembrane of mitochondria, which result in apoptosis
[22]. Several events following TBI, such as tumor necrosis factor (TNF) release, toll-like receptors (TLR) activation, inflammation, and reactive oxygen species (ROS) production, have the potential to activate necrosis, which involves the upstream assembly of the necroptosome complex formed by the interaction of receptor interacting protein kinase 1 and 3 (RIPK1 and 3) and the downstream RIPK3-mediated phosphorylation of mixed lineage kinase domain-like (MLKL) protein
[23], which result in necroptosis. Far more than that, the extent of cell loss following TBI has been correlated with cognitive deficits and long-term prognosis in both clinical and experimental studies
[24].
A correct assessment of the degree of injury is essential for the effective treatment of TBI. TBI severity is traditionally determined by several clinical indicators, including the consciousness state, Glasgow coma scale score, presence/duration of retrograde amnesia, and neuroimaging evidence
[25][26][25,26]. Neurological dysfunction can be divided into physical symptoms and neuropsychiatric symptoms. Neuropsychiatric dysfunctions, including cognitive impairments (executive dysfunction, attention disorder, or memory problems) and emotional/behavioral disorders (depression, anxiety, or sleep disorders)
[11][27][11,27] are common after TBI, typically lasting 7 to 10 days but sometimes months to years. Therefore, differences in neurological dysfunctions after TBI depend on the severity of brain injury as well as the characteristics of the underlying key brain regions (
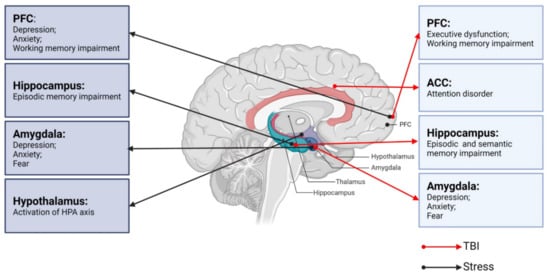
Figure 1).
Figure 1. Neurobiological links between TBI, stress, and neurological dysfunctions. PFC: prefrontal cortex; ACC: anterior cingulate cortex; TBI: traumatic brain injury.
2.1. TBI and Cognitive Impairments
Forms of cognitive impairments post-TBI range from difficulties with executive function, i.e., attention and problem solving, to deficits in information processing and short- and long-term memory
[28][29][28,29]. Previous research has shown that the cognitive functions, including memory, attention, and executive function, are resolved within 3 to 6 months after mild or moderate TBI
[30], whereas severe TBI can cause cognitive impairments for 6 months or longer
[28].
Funahashi
[31] describes executive function as a product of the coordinated operation of various processes to accomplish a particular goal in a flexible manner. Executive function is the province of the PFC, and executive dysfunction post-TBI is typically associated with frontal lobe injury. Executive function is mediated by a distributed network guided by the frontal lobe that includes the prefrontal sub-region, posterior cortex, and subcortical structures, such as the basal ganglia and ventral striatum
[32]. TBI causes the frontal lobe and subcortical structures, such as the cingulate gyrus, amygdala, striatum, and insula, to be particularly vulnerable
[33]. Accordingly, patients with PFC damage show alterations in judgment, organization, planning, and decision making. There are functional and anatomical links between the frontal cortex and striatum
[34]. Executive function relies on the efficient operation of cortical striatum circuits, which are often abnormal in cases of executive dysfunction
[35][36][35,36]. The striatum can be divided into the caudate nucleus, putamen, and nucleus accumbens (NAc)
[37]. The disruption of the striatum has been reported in various disorders involving executive dysfunction, such as Huntington’s disease
[38], multiple system atrophy (MSA)
[39], progressive supranuclear palsy
[40], and attention deficit hyperactivity disorder (ADHD)
[41]. Working memory is also an important part of executive function. Significant functional changes in the PFC circuit have been shown to reduce the large-scale patterns of brain activity, which is associated with working memory impairment
[42][43][42,43].
Attention is characterized as selectivity and intensity
[44]. Selectivity includes focalized attention with the inhibition of distractors and divided attention, which allows for the performance of two tasks simultaneously
[44]. Intensity consists of sustained attention allowing the person to maintain attention levels over prolonged periods of time, and to be kept alert
[44]. Impaired attention is one of the most common complaints of TBI survivors
[45][46][47][45,46,47]. Based on neuroimaging studies, some psychiatric symptoms have been shown to correspond to functional abnormalities in brain regions, such as selective attention being localized to the anterior cingulate cortex (ACC)
[48]. An insufficient activation of ACC can lead to reduced attention to detail and easy distraction. The ACC regulates the activity of the cortex and subcortical regions and influences the ability to control and coordinate their interactions
[49][50][51][49,50,51]. Interestingly, Sheth et al.
[52] reported resting state functional hyperconnectivity of the ACC in veterans with mild TBI (mTBI).
Several theories about learning and memory propose a stable link between the hippocampus and cortex that strengthens or consolidates memory
[53]. Memory impairments caused by TBI may thus be due to alterations in the physiological circuit involving the cortex and hippocampus. The division of memory into short-term and long-term memory remains controversial
[54], and memory can be alternatively divided into working, episodic, and semantic memory. The hippocampus is the core of episodic memory and the disruption of the dentate gyrus (DG), CA1, and CA3 regions is thought to be the main cause of episode memory deficits post-TBI
[42]. A prior study demonstrated a decreased hippocampal volume in mTBI patients with episodic memory impairment
[55]. The integrity of the hippocampus is also important for semantic memory. For example, Klooster et al.
[56] reported impoverished semantic memory in patients with hippocampal amnesia. Manns et al.
[57] assessed the semantic memory capacity of patients with hippocampus damage and found that semantic memory abilities were impaired—especially anterograde and retrograde memory.
2.2. TBI and Emotional/Behavioral Disturbances
The most common chronic emotional/behavioral disturbances that occur after TBI are depression, anxiety, and fear
[58]. The amygdala is a key brain region involved in emotional processing
[59] and amygdala damage is associated with emotional disorders similar to those that arise after TBI
[58][60][58,60]. The amygdala also plays an important role in recognizing facial emotion, such as fear, disgust, and anger
[61][62][61,62].
Depression, which is one of the most common chronic psychoses after TBI
[63], is thought to be closely related to changes in the amygdala. Depressed patients often have some negative symptoms, such as sleep disturbance, fatigue (anergia), difficulty with concentration, and anhedonia (apathy)
[64]. Studies have shown that depression is associated with increased activation in marginal regions, through which, the amygdala is richly associated with cortical regions
[65][66][65,66]. The effect of antidepressants is partly via effects on the coupling of the amygdala with other brain regions
[67]. In a functional neuroimaging study of patients with diffuse TBI
[68], the amygdala was found to process emotions and regulate behavioral and physiological responses to stressors
[69]. The PFC, as a significant center of thinking and behavior regulation, is also associated with depression
[70]. The PFC can be divided into medial PFC (mPFC) and dorsolateral PFC (dPFC)
[71]. Using diffusion tensor tractography, Jang et al. identified dPFC after TBI accompanied by depressive symptoms
[72]. The hippocampus is part of the limbic system and has nerve fiber connections with emotion-related brain regions such as the PFC and amygdala. A decrease in hippocampal volume has been observed in patients with TBI
[73]. In addition, hippocampal volume is associated with TBI injury severity and neuropsychological function
[74].
Anxiety is a common condition in which an anxious mood or state persists without an immediate threat. The amygdala, which plays a key role in regulating anxiety-related behaviors
[69], is composed of several parts. Of these, the basolateral amygdala (BLA) and central amygdala (CeA) are particularly important in anxiety management
[75][76][75,76]. The BLA consists of 80% pyramidal glutamate (Glu) neurons, and 20% γ-aminobutyric acid (GABA) neurons. The CeA, which encompasses the centrolateral (CeL) and centromedial (CeM) nuclei, consists of 95% GABAergic medium spiny neurons
[77]. The CeM, which is the main output region of the amygdala, mediates the autonomic and behavioral responses to anxiety via projections to the brainstem
[78]. The balance between excitation and inhibition determines the overall degree of amygdala excitability. The hypoactivity of GABAergic neurons and/or an increased activation of glutamatergic neurons leads to amygdala hyperexcitability that manifests as anxiety
[79]. Prior research suggested that TBI-induced anxiety-like behaviors were associated with increased glutamatergic neurons and decreased GABAergic neurons within the amygdala
[68]. Figueiredo et al.
[69] observed that animals exhibited increased anxiety-like behaviors 30 days after TBI.
The amygdala is also key for the acquisition and storage of fearful memory
[80]. The BLA is the main region associated with sensory inputs into the amygdala, while the CeM is known as the fear effector structure
[81]. The amygdala is also involved in regulating fear-related learning via interactions with other brain regions, such as the cortex and hippocampus. External stimuli information is processed through mechanisms inherent in the amygdala and by interactions with other brain regions to produce fear responses as an output and regulate fear responses
[82]. Similarly, Glu receptors and GABA receptors are essential for fear learning and memory
[83]. A recent study
[84] using single-nucleus RNA sequencing (snRNA-seq) demonstrated a significant increase in Decorin (a small leucinerich proteoglycans) expression in amygdala excitatory neurons after TBI, whereas the knockout of
Decorin alleviated TBI-related fear conditioning.
Overall, immediate or secondary pathological changes following TBI can lead to abnormal cognitive and emotional function. Through clinical interventions, most non-severe TBI patients recover in a short period without any sequelae. However, some non-severe TBI patients still show delayed and even severe neurological abnormalities
[85][86][85,86]. Previous studies have found that stress may aggravate or improve neurological dysfunctions following non-severe TBI
[87][88][87,88].
3. Neurobiological Links between Stress and Key Brain Regions
All organisms maintain a complex dynamic equilibrium or homeostasis that is constantly challenged by internal or external stimuli, which are termed stressors
[89]. Physiological stress is beneficial to the body in that the body can quickly adapt to changes in internal and external environmental factors. However, pathological stress that is intense and persistent is harmful to the body and can cause physical and mental dysfunction, resulting in many negative adaptation reactions
[90]. The brain processes external information and determines the necessary behavior and physiological responses, whether adapting or overloading. As an organ, the brain changes in response to acute and chronic stress
[91], and stress hormones can have protective or destructive effects on the brain. Studies have shown that moderate stress facilitated classical conditioning and associative learning
[92], in contrast to the chronic stress-induced deficits in spatial and contextual memory and attention
[93]. It is noteworthy that different stress paradigms can have a psychological influence or physical impact simultaneously. Psychological stressors may include social order conflicts and competition for resources, as well as restraint and immobilization with accompanying anxiety and fear
[94]. Methods of physical stress include, but are not limited to, a lack of food or water, handling, and surgical procedures
[95]. The HPA axis and LC-NE system play major roles in these stress responses. Neural circuits between different brain regions, including the hippocampus, amygdala, PFC, and hypothalamus, also play pivotal roles in responding to stress (
Figure 1)
[96].
3.1. Stress and the HPA Axis
The activation of the HPA axis is the primary hormonal response to stress. The initiation of the HPA axis is controlled by corticotropin-releasing hormone (CRH) neurons of the paraventricular nucleus (PVN)
[97]. CRH and arginine vasopressin (AVP), which are released by PVN through the pituitary portal to the pituitary gland, act together, acting on the pituitary gland to promote the release of adrenocorticotropic hormone (ACTH) via the circulatory system to the adrenal cortex, thereby promoting the synthesis and release of glucocorticoids (GCs), which act on the body’s organ systems to adapt to changes in the internal and external environment
[98]. GCs primarily bind to two receptors in the brain, namely the mineralocorticoid receptor (MR) and GC receptor (GR). The activation of these receptors alters the gene expression profiles slowly and persistently, ultimately affecting brain function
[97][99][97,99]. A prior study showed that GCs are beneficial for short-term adaptation, but that long-term administration can cause severe damages
[100].
3.2. Stress and the LC-NE System
The LC in the brainstem contains NE-synthesizing neurons that send diffuse projections throughout the central nervous system (CNS). The LC-NE system plays a major role in behavioral and autonomic responses to stress. During a stressful period, LC-NE neurons supply NE across the CNS to modulate the central stress response
[101][102][101,102]. NE acts on different adrenal receptors (α
1, α
2, and β) and exerts a powerful neuroregulatory function. NE has a higher affinity for α
2-adrenergic receptors and a lower affinity for α
1- and β-adrenergic receptors
[103]. The LC is involved in the stress response mainly through β receptors located in the BLA
[104].
3.3. Stress and the PFC
The mPFC is primarily composed of glutamatergic pyramidal neurons (PNs)
[105]. The mPFC PNs that orchestrate stress responses are tightly controlled by a complex network of GABAergic interneurons
[106]. Although there are several groups of interneurons, the majority of GABAergic interneurons express parvalbumin (PV) and are thus termed PV neurons. Somatostatin (SST) neurons are also thought to regulate the Glu output from the dendritic trees of PNs through synaptic contact
[107]. An imbalance between excitatory and inhibitory (E/I) neurotransmission is thought to be the basis for various neuropsychiatric disorders
[108]. These interneurons express GRs and have the ability to integrate systemic stress signals. GRs are bound during acute stress, increasing soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein complexes (which mediate synaptic vesicles to fuse with the anterior membrane) in the presynaptic membrane
[109][110][109,110]. Therefore, acute stress increases the excitability of glutamatergic neurons, as shown by extracellular Glu, postsynaptic membrane N-methyl d-aspartate receptor (NMDAR), and alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) expressions, and increases in NMDA and AMPA-mediated excitatory currents. Animals studies have shown that acute exposure to stress or the administration of GCs increased the Glu release from the PFC
[110][111][110,111]. Using microdialysis, it has been shown that exposing rats to tail-pinch, restraint, or forced-swim stress induces a marked, transient increase in extracellular Glu levels in the PFC
[107]. Conversely, repeated stress has been shown to inhibit Glu delivery in the PFC by promoting the degradation of Glu receptors in juvenile rats
[111].
The PFC has extensive neuronal connections with other brain regions that regulate behavior, cognition, and emotions
[112]. Catecholaminergic neurons projections to the cerebral cortex stem from two main sources, namely NE neurons of the LC in the brainstem and dopamine (DA) neurons of the ventral tegmental area (VTA) in the midbrain. The PFC is a main cortical target of both NE and DA innervations
[113]. NE and DA each have an ‘inverted U’-shaped influence on working memory, such that either too little or too much impairs PFC function
[114][115][114,115]. A previous study showed that α
1-adrenoceptor stimulation in the PFC contributes to stress-induced cognitive impairments
[116]. The low NE level present under control (non-stress) conditions optimizes working memory by engaging α
2A-receptors, whereas the high NE level during stress impairs PFC function by stimulating lower-affinity α
1-receptors and β
1-receptors
[117]. An excessive activation or blocking of dopamine D1 receptors (D1Rs) during working memory stress can both lead to working memory deficits
[114]. Memory deficits due to the excessive activation of D1R can be prevented by D1R antagonists
[118], whereas spatial working memory deficits mediated by an increased D1R density are improved by D1R agonists
[119]. Under normal circumstances, the extensive connections of PFC coordinate brain activity and regulate the catecholamine input.
3.4. Stress and Hippocampus
The hippocampus is rich in GRs and MRs
[120], making it a key regulatory region of the HPA axis. Stress has a great impact on excitatory transmission and the synaptic plasticity of the hippocampus
[121]. Excitatory amino acids and NMDAR play important roles in episodic memory function, which is dominated by the hippocampus. Excitatory amino acids produce long-term potentiation (LTP) on synapses
[122]. Thus, the plasticity of synaptic connections in the hippocampus and LTP are the basis of learning and memory. LTP production relies on synaptic connections between cells in the CA1 and CA3 regions of the hippocampus, which, in turn, depend on Glu as a neurotransmitter
[123]. During stress, GCs increase and stimulate Glu release from the hippocampus, which, in turn, inhibits DG proliferation
[124]. Studies have shown that exposure to predator odor (2,4,5-trimethythiazole, TMT) causes a stress response in rats, as demonstrated by elevated adrenal steroid levels, DG excitation, and the rapid inhibition of DG proliferation
[125]. CA3 dendritic atrophy is suppressed when an excitatory input pathway is damaged. Antagonism with NMDAR can inhibit stress-induced CA3 dendritic atrophy
[126]. Interestingly, morphological damage of the CA3 region was reversed within 21 days in a rat model after the end of chronic stress
[127]. Chronic stress can also produce CA1 apical dendritic retraction, although stressors tend to be more severe than what is needed to produce an apical dendritic retraction in the CA3 region
[128].
3.5. Stress and Amygdala
The amygdala plays a key role in physiological and behavioral responses to stress and is characterized by high inhibitory tension mediated by GABA at rest. Stress causes hyperactivity of the amygdala, which is often accompanied by a reduction in inhibition controls
[129]. Under physiological conditions, mPFC exerts top-down inhibitory control over amygdala activity, limiting its output and thereby preventing an inappropriate expression of emotions. Under stress conditions, the amygdala activates stress pathways in the hypothalamus and brainstem to induce high levels of NE and DA release, thereby impairing PFC regulation but strengthening amygdala function
[130]. In such cases, the PFC control of stress becomes defective, resulting in aberrant amygdala activation and deficits in emotion and behavior
[131]. Thus, during stress, the orchestration of the brain’s response patterns switches from the slow, thoughtful PFC regulation to the reflexive, rapid emotional regulation mediated by the amygdala and related subcortical structures
[11].
Stress causes the remodeling of amygdala neuronal projections
[132]. In contrast to stress-induced dendritic retraction seen in the hippocampus, projecting neurons within the BLA showed persistent dendritic hypertrophy after chronic stress, but dendritic contractions after acute stress
[7]. Thus, the morphology of the amygdala and hippocampus showed opposite adjustments after chronic stress. In contrast, Glu is enhanced in both the amygdala and hippocampus after stress. The increase in Glu levels activates NMDAR in BLA, thereby delaying the increase in synaptic spines. A prior study showed that the GR agonist dexamethasone (DEX) enhanced fear resolution via a dose-dependent regulation of the methylation of the GR partner FK506-binding protein 5 (FKBP5) in the BLA
[133]. The amygdala is also a major extrahypothalamic source of corticotropin releasing factor (CRF)-containing neurons and has high expression levels of the two cognate CRF receptors. During chronic stress, the repeated activation of CRF receptors leads to an increased NMDAR-mediated Ca
2+ inflow
[134], which inhibits the polymerization of tubulin dimers responsible for microtubule and neurite elongation. If Ca
2+ is sustained at high levels, microtubules and microfilaments will be depolymerized to trigger dendritic regression
[135].
Thus, under stress conditions, the HPA axis and LC-NE systems act with central GRs through the final metabolites GCs and NE. The activation of MRs and adrenal receptors affects the balance of excitatory and inhibitory neurons in the PFC, hippocampus, and amygdala, as well as neuronal plasticity, ultimately affecting working memory, emotions, and other neurological functions. Taken together, these findings indicate that both stress and TBI can lead to deficits in the corresponding brain regions.