You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by eugenio Bertelli.
Idiopathic epiretinal membranes (iERMs) are fibrocellular sheets of tissue that develop at the vitreoretinal interface. The iERMs consist of cells and an extracellular matrix (ECM) formed by a complex array of structural proteins and a large number of proteins that regulate cell–matrix interaction, matrix deposition and remodelling. Many components of the ECM tend to produce a layered pattern that can influence the tractional properties of the membranes.
- idiopathic epiretinal membranes
- extracellular matrix
- ITGB1
1. Introduction
The vitreoretinal interface is the border region where the vitreous meets the internal limiting membrane (ILM) of the retina [1]. In general, the vitreous does not adhere strongly to the ILM with the notable exception of a few important areas including the vitreous base, a perifoveal rim and the optic papilla [1,2][1][2]. The vitreoretinal interface is the site where a series of pathologies such as posterior vitreous detachment, macular holes (MH)s, vitreomacular traction and epiretinal membranes (ERM)s can develop [3]. ERMs, in particular, are formed by a fibrocellular layer of tissue that can seriously affect vision when developing and retracting in front of the macula [4]. ERMs are generated secondarily to systemic or local diseases (e.g., diabetes, retinal tears), or they can develop without any apparent reason. In the latter case, they are referred to as idiopathic ERMs (iERM)s. Although the cellular component of ERMs has been the object of several investigations and many molecular markers have been employed for cell identification, the source of ERM cells is a matter that still appears far from being solved [5,6,7,8,9,10,11,12][5][6][7][8][9][10][11][12]. Probably due to a process of transdifferentiation, ERM cells take on a myofibroblastic phenotype with the expression of α-smooth muscle actin (α-SMA) [5,6,7,8,9,10,11,12][5][6][7][8][9][10][11][12] and the ability to deposit new extracellular matrix (ECM) [11,12][11][12].
2. ECM Components of iERMs
The ECM of iERMs encompasses many collagens, basement membrane proteins, proteoglycans, GAGs, as well as some adhesive and matricellular proteins. In addition, collagens IX, XII, XIV, XVIII and XXII have been found by proteomic profiling [13].2.1. Collagens
The major component of the iERM ECM is certainly represented by collagen. So far, only collagens I to VII have been detected in iERMs by immunohistochemistry. In addition, collagens IX, XII, XIV, XVIII and XXII have been found by proteomic profiling [13].2.1.1. Collagen I
Collagen I has been repetitively reported in iERMs [12[12][14][15][16][17],18,19,20,21], and it is certainly shared with other types of ERMs [22,23,24][18][19][20]. As collagen I is a hallmark of all fibrotic responses, one might expect to find it in all ERMs, and this was the case in many studies [12,20,21][12][16][17]. However, some investigators also observed a certain number of iERMs apparently lacking collagen I [18,19][14][15]. Proteomic profiling confirmed the presence of α1(I) and α2(I) collagen chains [13], and gene expression analysis showed that COL1A1 gene is expressed by iERM cells [25,26][21][22]. Though with some restraints, an attempt to characterize cells responsible for collagen I deposition has recently taken place by confocal microscopy with the identification of glial fibrillary acid protein (GFAP)-/vimentin+/αSMA+/heat shock protein 90+ myofibroblast-like cells with collagen I-immunoreactive intracytoplasmic bodies [12]. One shortcoming of this approach, however, is that the collagen I intracellular immunoreactivity can be due to matrix deposition as well as to collagen reabsorption. Previous studies carried out in ERMs from eyes affected by PVR have shown that only a few collagen I-immunoreactive cells are GFAP+ elements, for the most part being cytokeratin+ epithelial- and fibroblast-like cells or cytokeratin-/αSMA+ fibroblast-like cells [23,24][19][20].2.1.2. Collagen II
Collagen II is a well-known constituent of the vitreous [1], and it has been reported in iERMs on several occasions [18,19,27][14][15][23]. Nonetheless, its presence does not appear a constant feature, and when detected, collagen II-immunoreactivity occupies a residual part of histologic sections [19,27][15][23]. According to Okada et al. [18][14], however, when iERMs are removed still associated with the ILM, a thin layer of collagen II-immunoreactive ERM can be seen adherent to the ILM. Thus, the presence or absence of collagen II in histologic sections could be related to the removal of the ILM along with the iERM or to the more or less complete detachment of the iERM from the ILM. On the other hand, the more sensitive proteomic approach demonstrated the presence of an α1(II) collagen chain in all tested samples [13]. ERMs from PVR eyes also show an inconstant expression of collagen II which is frequently restricted to small portions of the specimens [23][19]. Therefore, its presence could be the result of residual fragments of the vitreous cortex trapped within the developing ERMs more than the outcome of novel deposition [18][14]. This interpretation is corroborated by real-time polymerase chain reaction (RT-PCR) experiments that did not find any relevant COL2A1 gene expression in PVR ERMs [28][24].2.1.3. Collagen III
Collagen III is usually found within collagen I fibrils which are mostly heterotypic fibrils. When the ratio of the two collagens is in favour of collagen III, collagen fibrils assume the aspect of reticular fibres [29][25], but when the ratio is in favour of collagen I, fibrils are plainly referred to as collagen I fibrils. Although collagen III in iERMs has received little attention, when searched, it was found with the same frequency as collagen I [18,21][14][17]. Similar results have been observed in ERMs from PVR eyes [22[18][19],23], where the areas occupied by collagen III and collagen I on histologic sections matched [23][19]. As collagens I and III co-polymerize within the same fibril, the GFAP−/vimentin+/αSMA+/heat shock protein 90+ myofibroblast-like cells that are candidate for collagen I production [12] are also reasonably responsible for collagen III deposition. Yet, it is puzzling how proteomic profiling of iERMs did not detect collagen III [13]. On the other hand, in keeping with collagen I/III co-localization within the same fibrils, COL3A1 was found expressed in ERMs from PVR along with COL1A1 and COL1A2 [28][24].2.1.4. Collagen IV
Collagen IV is usually considered a basement membrane component [30][26]. In some instances, collagen IV is also found in interstitial deposits, either in physiologic conditions [31[27][28],32], or, more frequently, in fibrotic diseases [33,34][29][30]. Collagen IV is a constant constituent of iERMs [12[12][14][15][16][23],18,19,20,27], as well as of ERMs from PVR eyes [22,23,35][18][19][31]. A diffuse and considerable intense staining, which does not fit with a mere basement membrane-related immunoreactivity, has been reported by many investigators [12,19,20,21,27][12][15][16][17][23]. A study of collagen IV distribution in iERMs has been recently carried out by confocal microscopy showing that this collagen is deposited interstitially, intermingled with collagen I, with a staining intensity that progressively increases towards the vitreous side of the membrane. On the other hand, collagen IV also forms continuous or discontinuous basement membrane-like structures in close association with cells which differ from classic basement membranes by the presence of collagen I [20][16]. Transmission electron microscopy confirms interstitial condensations of collagen IV-immunoreactive material that are frequently infiltrated by collagen I fibrils (Figure 1). In this respect, collagen IV-immunoreactive areas mostly also co-localize with collagen III and collagen VI [12,20,21][12][16][17]. Anti-collagen IV antibody-labelled material can even be observed within intracytoplasmic vesicles or lysosome-like bodies, suggesting active remodelling of the ECM by ERM cells.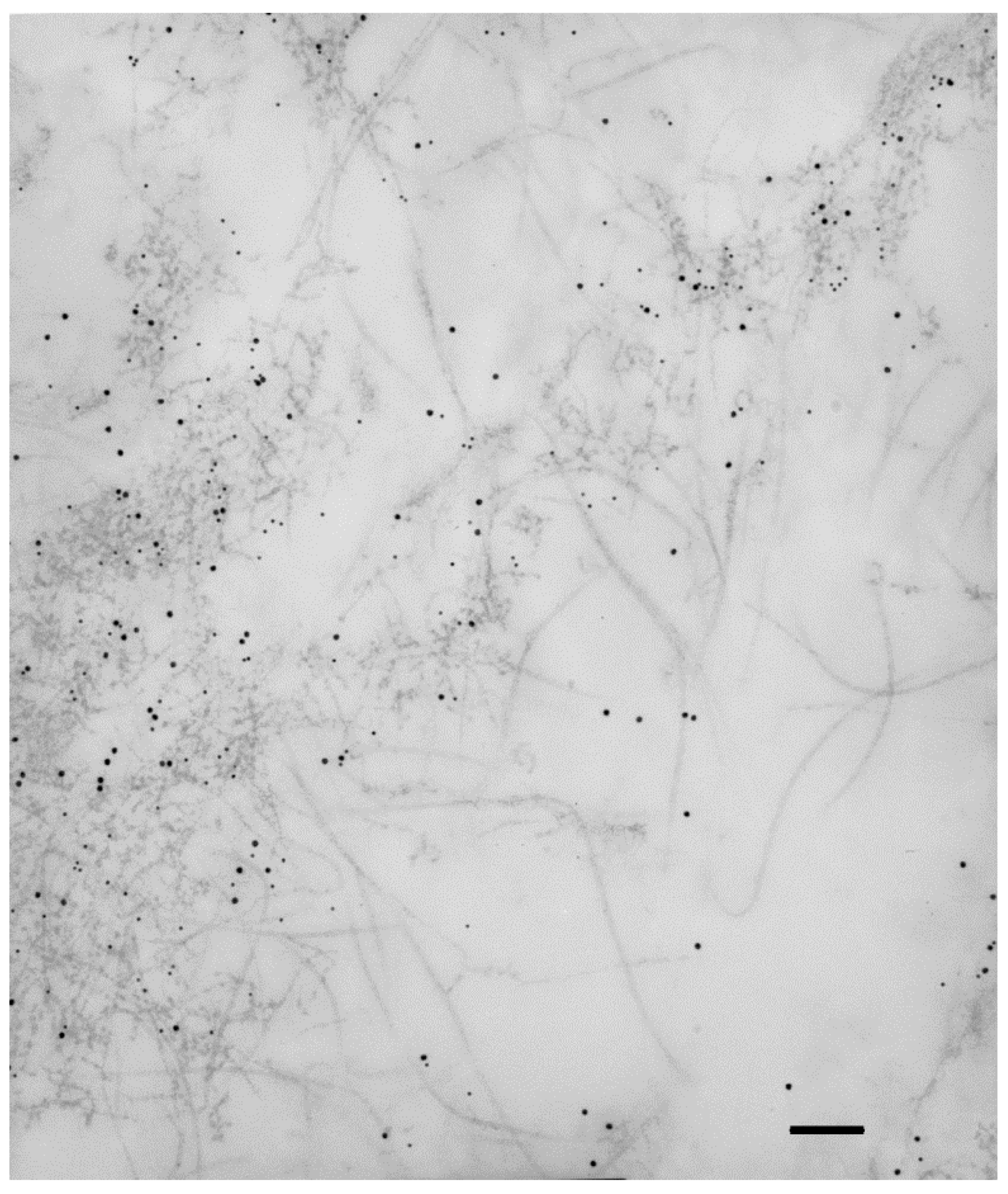
Figure 1. Collagen IV immunoreactivity is not restricted to basement membranes. Extracellular matrix of an iERM double-labelled with anti-collagen IV and anti-collagen I antibodies which were unveiled with the appropriate gold-conjugated secondary antibodies. Collagen IV labelling (10 nm gold particles) is restricted to condensations of fuzzy material which is infiltrated by collagen I immunoreactive fibrils (15 nm gold particles). Magnification bar = 180 nm.
2.1.5. Collagen V
Collagen V is one of the least studied collagens among those detected in ERMs. Actually, to our knowledge, its presence has been assessed by immunohistochemistry only once in ERMs from PVR eyes where it was constantly found [22][18]. However, collagen V is known to form heterotypic fibrils with collagen I. The ratio between collagen V and collagen I is considered inversely correlated with collagen I fibril diameter [36][32]. In other words, collagen V is a key regulator of collagen I fibril size: the higher the amount of collagen V, the smaller collagen I fibrils are. On the other hand, the presence of collagen V is not supported by proteomic analysis which did not consistently find COL5A1 and COL5A2 in iERMs [13].2.1.6. Collagen VI
Collagen VI has been detected in iERMs by several investigators [11,19,20,21][11][15][16][17]. Its expression has been found particularly relevant only in a subtype of iERMs, the so-called cellophane macular reflex membranes, whereas it is absent or almost negligible in the preretinal macular fibrosis subtype [19][15]. In contrast to iERMs, collagen VI is not present in ERMs associated with MHs [37][33]. Collagen VI co-localizes with collagen IV in large areas of iERMs [20][16]. However, the distribution of the two collagens does not match perfectly as immunoreactive areas for just one of the two collagens are also present [21][17]. Whereas proteomic profiling of iERMs has not confirmed the presence of collagen VI [13], gene expression analysis did find COL6A1 mRNA [26][22].2.1.7. Collagen VII
A recent addition to the list of collagens associated with iERMs is collagen VII that has been detected in two specimens [38][34]. However, proteomic analysis did not reveal collagen VII expression in iERMs [13], and RT-PCR did not detect COL7A1 mRNA in ERM in PVR eyes [28][24].2.1.8. Other Collagens
Some additional collagens (IX, XII, XIV, XVIII, XXII) have been identified in iERMs exclusively by proteomics [13]. Collagens IX, XII, XIV and XXII belong to the Fibril-Associated Collagen with Interrupted Triple helices (FACIT) family. Collagen IX is found on the surface of collagen II fibrils, whereas collagens XII and XIV associate with the surface of collagen I fibrils [39][35]. Thus, even though they have not been specifically investigated, collagen IX should co-localize with collagen II, whereas collagens XII and XIV should match exactly collagen I fibrils distribution. Collagen XXII, in spite of the FACIT molecular structure, is found associated with microfibrils, possibly fibrillin or fibronectin (FN) [40][36]. It has a very specific pattern of expression, being found at sites of tissue transitions such as hair follicles, myotendinous junctions (skeletal muscles, ciliary body, heart) and muscle insertion to the ribs [40][36]. It is worth noting that its expression is mostly located where tractional forces are exerted. Collagen XVIII is a basement membrane-associated collagen [41][37]. As iERMs contain several basement membrane proteins (see Section 3.2), it is conceivable that collagen XVIII may co-localize with them. Interestingly, collagens IX, XII and XVIII are also proteoglycans as they have GAG side chains. Collagens IX and XII are associated with chondroitin sulfate, whereas almost half the molecular weight of collagen XVIII is due to heparan sulfate side chains [41][37].2.2. Basement Membrane Proteins and Their Assembly in LNCP Matrix
In addition to collagens IV and XVIII (see Section 3.1), basement membrane proteins include laminins, nidogens/entactins and heparan sulfate proteoglycans.2.2.1. Laminins
Laminins are trimeric molecules comprising α, β and γ chains. They assemble generating at least 16 different isoforms. Current nomenclature identifies each laminin isoform with a three-digit number which refers to the α, β and γ chains. Thus, for instance, laminin 521 is the isoform composed by laminin chains α5, β2 and γ1. The presence of laminin in iERMs has been frequently reported [19[15][16][17],20,21], and its chain composition has been studied. By immunofluorescence, only laminin α1, β1 and γ1 have been easily detected. Laminin α5 has also been found, though apparently far less represented [21][17]. Based on these results, iERMs apparently contain laminin 111 and, possibly, minor amounts of laminin 511 [21][17]. However, proteomic analysis has found a more complex pattern of laminin expression, including α1, α2, α4, α5, β1, β2 and γ1 chains [13]. A wider range of laminin isoforms, therefore, is probably expressed in iERMs. Even though it can be found alone [20][16], laminin mostly co-localizes with collagen IV and nidogen/entactin [21][17]. Co-localization of three major components of basement membranes suggests that the condensations of collagen IV observed in iERMs could be an example of the so-called laminin/nidogen/collagen IV/perlecan (LNCP) matrix (see Section 3.2.4).2.2.2. Nidogens/Entactins
Nidogen/entactin 1 and nidogen/entactin 2 are proteins typically expressed in basement membranes. They are thought to link laminin and collagen IV lattices together within planar basement membranes [30][26]. Nidogens/entactins immunolocalization in iERMs found nidogen/entactin 1 co-localized with collagen IV and laminin [21][17] supporting the existence of LNCP matrix deposits. Proteomic analysis has confirmed the presence of both nidogens/entactins proteins [13].2.2.3. Heparan Sulfate Proteoglycans
Three heparan sulfate proteoglycans, perlecan, agrin and collagen XVIII, are found in basement membranes [42][38]. All of them have been convincingly detected by proteomic profiling in iERMs [13]. Heparan sulphate proteoglycans distribution could match LNCP matrix deposits though their immunolocalization has never been assessed.2.2.4. LNCP Matrix
The LNCP matrix is an extracellular matrix polymer containing laminin, nidogen, collagen IV and perlecan, which does not form planar basement membranes [43][39]. Though more common in drosophila, examples of LNCP matrix can be found in human tissues as well, including placenta, specifically the foetus–mother interface [32][28], chondrocyte pericellular matrix [44][40] and the corneal stroma [31][27]. As previously reported, laminin, nidogen and collagen IV colocalize in iERMs [21][17]. Proteomic analysis also identified perlecan in these membranes [13], though its exact distribution has never been assessed. It is possible, therefore, that laminin-, nidogen- and collagen IV-immunoreactive material in iERMs may indeed represent deposits of LNCP matrix. In iERMs, however, collagen IV also co-localizes with collagen I, a feature that likely differs from other examples of LNCP matrix.2.3. Proteoglycans
In addition to being associated with basement membranes, proteoglycans represent fundamental constituents of the ECM ground substance. Yet, they are certainly far less studied than proteins. As a result, investigations on proteoglycans are also extremely limited in ERMs. Perlecan, decorin and tenascin have been detected in PVR-associated ERMs [22,35,45,46][18][31][41][42]. Opticin has been also observed in some samples of PVR showing a variable pattern of distribution [47][43]. In iERMs, proteoglycans have been studied only by proteomic profiling. In addition to the previously mentioned heparan-sulfate proteoglycans associated with basement membranes (see Section 3.2.3), a wide variety of proteoglycans including decorin, biglycan, fibromodulin, prolargin (PRELP), opticin, chondroadherin-like protein and podocan have been found [13].2.4. Other Proteins
A few additional proteins have been investigated in the ECM of ERMs. They include microfibrillar, adhesive and matricellular proteins.2.4.1. Fibrillin
Fibrillin is a microfibrillar protein of the elastic system. It is found as a component of oxytalan, elaunin and elastic fibers. The elastic system of the ECM has been studied by light and transmission electron microscopy only in samples of PVR where oxytalan fibers have been observed either in subretinal or in ERMs [48][44]. In iERMs, proteomic analysis constantly found fibrillin 1 [13].2.4.2. Fibronectin
FN is an adhesive protein of the ECM that is expressed in all ERMs, including iERMs [18,19,21,23,35,46,49,50,51][14][15][17][19][31][42][45][46][47]. In iERMs, FN is usually found close to the cell layer (see Section 4) where it partially co-localizes with collagen IV [18,21][14][17]. FN can exist as a soluble or a cell-associated form. The latter form differs from the soluble one by the presence of highly conserved alternatively spliced FN type III repeats termed Extra Domain A (EDA) and Extra Domain B (EDB). Both soluble and cellular forms co-exist in PVR-associated ERMs, [52][48] and the presence of cellular FN has been confirmed by confocal microscope detection of FN EDA. Interestingly, FN EDA immunofluorescence appears in close relationships with myofibroblasts. Alignment of myofibroblast stress fibers with FN fibrils suggests anchorage through fibronexus [7]. Cell-associated FN is also actively produced in iERMs as demonstrated by proteomic analysis and by the presence of EDB FN mRNA [13,25][13][21].2.4.3. Vitronectin
Vitronectin is another adhesive protein of the ECM. Its immunolocalization, to our knowledge, has been studied only in ERMs associated with PVR [49[45][47],51], where it is observed pericellularly along with FN, though FN staining appears more diffuse throughout membranes [49][45]. In iERMs, vitronectin has been detected by proteomic analysis [13].2.4.4. Thrombospondin 1
Thrombospondin 1 is an ECM protein with multiple functions. It is a matricellular protein and, as such, it is highly expressed upon tissue injury [53][49]. Thrombospondin 1 has been found expressed only in PVR-associated ERMs [50,54][46][50].2.4.5. Osteonectin/SPARC
An important role in the development of PVR-associated ERMs has been proposed for osteonectin [55][51]. Osteonectin is a matricellular protein that is also called Secreted Protein Acidic and Rich in Cysteine (SPARC). It has been observed predominantly within cells in retinas affected by PVR [55][51]. A convincing demonstration of osteonectin in iERMs is lacking, even by proteomics [13].2.4.6. Periostin
Periostin is another matricellular protein that is highly expressed in PVR-associated ERMs. Its highest expression, however, is found in ERMs associated with PDR. Similar to osteonectin, periostin has been shown within ERM cells [56][52]. Proteomic profiling did not detect thus protein in iERMs [13].2.5. Glycosaminoglycans (GAG)
GAG constituents of ERMs are a completely uncharted territory. Interestingly, intravitreal injection of chondroitin-6-sulfate triggers the development of experimental ERMs [57][53]. One might expect that this finding should have prompted researchers to investigate GAG composition of ERMs, either idiopathic or secondary. Surprisingly, this was not the case.References
- Bertelli, E. Aqueous Humor, Lens, Ciliary Zonule, Vitreous. In Anatomy of the Eye and Human Visual System; Bertelli, E., Ed.; Piccin Nuova Libraria: Padova, Italy, 2019; pp. 249–267.
- Tosi, G.M.; Marignani, D.; Romeo, N.; Toti, P. Disease pathways in proliferative vitreoretinopathy: An ongoing challenge. J. Cell Physiol. 2014, 229, 1577–1583.
- Patronas, M.; Kroll, A.J.; Lou, P.L.; Ryan, E.A. A review of the vitreoretinal interface pathology. Int. Ophthalmol. Clin. 2009, 49, 133–143.
- Folk, J.C.; Adelman, R.A.; Flaxel, C.J.; Hyman, L.; Pulido, J.S.; Olsen, T.W. Idiopathic epiretinal membrane and vitreomacular traction preferred practice pattern (®) guidelines. Ophthalmology 2016, 123, P152–P181.
- Hiscott, P.S.; Grierson, I.; McLeod, D. Natural history of fibrocellular epiretinal membranes: A quantitative, autoradiographic, and immunohistochemical study. Br. J. Ophthalmol. 1985, 69, 810–823.
- Sramek, S.J.; Wallow, I.H.; Stevens, T.S.; Nork, T.M. Immunostaining of preretinal membranes for actin, fibronectin and glial fibrillary acidic protein. Ophthalmology 1989, 96, 835–841.
- Bochaton-Piallat, M.-L.; Kapetanios, A.D.; Donati, G.; Redard, M.; Gabbiani, G.; Pournaras, C.J. TGFβ1, TGFβ receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2336–2342.
- Cabay, L.; Willermain, F.; Bruyns, C.; Verderbout, J.M.; Witta, Y.; Baffi, J.; Velu, T.; Libert, J.; Caspers-Velu, L.; Maho, A.; et al. CX3CR4 expression in vitreoretinal membranes. Br. J. Ophthalmol. 2003, 87, 567–569.
- Zhao, F.; Gandorfer, A.; Haritoglou, C.; Scheler, R.; Schaumberger, M.M.; Kampik, A.; Schumann, R.G. Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome. Analysis of flat-mounted internal limiting membrane specimens. Retina 2013, 33, 77–88.
- Schumann, R.G.; Gandorfer, A.; Ziada, J.; Scheler, R.; Schaumberger, M.M.; Wolf, A.; Kampik, A.; Haritoglou, C. Hyalocytes in idiopathic epiretinal membranes: A correlative light and electron microscopic study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1887–1894.
- Bu, S.-C.; Kuijer, R.; van der Worp, R.J.; Postma, G.; de Lavalette, R.; Li, X.-R.; Hooymans, J.M.M.; Los, L.I. Immunohistochemical evaluation of idiopathic epiretinal membranes and in vitro studies on the effect of TGFβ on Müller cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6506–6514.
- Tosi, G.M.; Regoli, M.; Altera, A.; Galvagni, F.; Arcuri, C.; Bacci, T.; Elia, I.; Realini, G.; Orlandini, M.; Bertelli, E. Heath shock protein 90 involvement in the development of idiopathic epiretinal membranes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 34.
- Christakopoulos, C.; Cehofski, L.J.; Christensen, S.R.; Vorum, H.; Honore, B. Proteomics reveals a set of highly enriched proteins in epiretina membrane compared with inner limiting membrane. Exp. Eye Res. 2019, 186, 107722.
- Okada, M.; Ogino, N.; Matsumura, M.; Honda, Y.; Nagai, Y. Histological and immunohistochemical study of idiopathic epiretinal membrane. Ophthalmic Res. 1995, 27, 118–128.
- Kritzenberger, M.; Junglas, B.; Framme, C.; Helbig, H.; Gabel, V.P.; Fuchshofer, R.; Tamm, E.R.; Hillenkamp, J. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 2011, 58, 953–965.
- Regoli, M.; Tosi, G.M.; Neri, G.; Altera, A.; Orazioli, D.; Bertelli, E. The peculiar pattern of type IV collagen deposition in epiretinal membranes. J. Histochem. Cytochem. 2020, 68, 149–162.
- Altera, A.; Tosi, G.M.; Regoli, M.; De Benedetto, E.; Bertelli, E. The extracellular matrix complexity of idiopathic epiretinal membranes and the bilaminar arrangement of the associated internal limiting membrane in the posterior retina. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2559–2571.
- Jerdan, J.A.; Pepose, J.S.; Michels, R.G.; Hayashi, H.; De Bustros, S.; Sebag, M.; Glaser, B.M. Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology 1989, 96, 801–810.
- Morino, I.; Hiscott, P.; McKechnie, N.; Grierson, I. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br. J. Ophthlamol. 1990, 74, 393–399.
- Guenther, S.R.; Schumann, R.G.; Hagenau, F.; Wolf, A.; Priglinger, S.G.; Vogt, D. Comparison of surgically excised premacular membranes in eyes with macular pucker and proliferative vitreoretinopathy. Curr. Eye Res. 2019, 44, 341–349.
- George, B.; Chen, S.; Chaudhary, V.; Gonder, J.; Chakrabarti, S. Extracellular matrix proteins in epiretinal membranes and in diabetic retinopathy. Curr. Eye Res. 2009, 34, 134–144.
- Coltrini, D.; Belleri, M.; Gambicorti, E.; Romano, D.; Morescalchi, F.; Chandran, A.M.K.; Calza, S.; Semeraro, F.; Presta, M. Gene expression analysis identifies two distinct molecular clusters of idiopathic epiretinal membranes. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165934.
- Snead, D.R.J.; Cullen, N.; James, S.; Poulson, A.V.; Morris, A.H.C.; Lukaris, A.; Scott, J.D.; Richards, A.J.; Snead, M.P. Hyperconvolution of the inner limiting membrane in vitreomaculopathies. Graefes Arch. Clin. Exp. Ophthamol. 2004, 242, 853–862.
- Asato, R.; Yoshida, S.; Ogura, A.; Nakam, T.; Ishikawa, K.; Nakao, S.; Sassa, Y.; Enaida, H.; Osima, Y.; Ikeo, K.; et al. Comparison of gene expression profile of epiretinal membranes obtained from eyes with proliferative vitreoretinopathy to that of secondary epiretinal membranes. PLoS ONE 2013, 8, e54191.
- Fleischmajer, R.; Jacobs, I.I.L.; Perfish, J.S.; Katchen, B.; Schwartz, E.; Timpl, R. Immunochemical analysis of human kidney reticulin. Am. J. Pathol. 1992, 140, 1225–1235.
- Yurchenko, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911.
- Pratt, B.M.; Madri, J.A. Immunolocalization of type IV collagen and laminin in nonbasement membrane structures of murine corneal stroma A light and electron microscopy. Lab. Investig. 1985, 52, 650–656.
- Oefner, C.M.; Sharkey, A.; Gardner, L.; Critchley, H.; Oyen, M.; Moffett, A. Collagen type IV at the fetal-maternal interface. Placenta 2015, 36, 59–68.
- Sharma, A.K.; Mauer, S.M.; Kim, Y.; Michael, A.F. Interstitial fibrosis in obstructive nephropathy. Kidney Int. 1993, 44, 774–788.
- Urushiyama, H.; Terasaki, Y.; Nagasaka, S.; Terasaki, M.; Kunugi, S.; Nagase, T.; Fukuda, Y.; Shimizu, A. Role of α1 and α2 chains of type IV collagen in early fibrotic lesions of idiopathic interstitial pneumonias and migration of lung fibroblasts. Lab. Investig. 2015, 95, 872–885.
- Ioachim, E.; Stefaniotou, M.; Gorezis, S.; Tsanou, E.; Psilas, K.; Agnantis, N.J. Immunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2005, 15, 384–391.
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–227.
- Bu, S.C.; Kuijer, R.; van der Worp, R.J.; Huiskamp, E.A.; Renardel de Lavalette, V.W.; Li, X.R.; Hooymans, J.M.; Los, L.I. Glial cells and collagens in epiretinal membranes associated with idiopathic macular holes. Retina 2014, 34, 897–906.
- Wullink, B.; Pas, H.H.; Ven der Worp, R.J.; Kuijer, R.; Los, L.I. Type VII collagen expression in the human vitreoretinal interface, corpora amylacea and inner retinal layers. PLoS ONE 2015, 10, e0145502.
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978.
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Breucknes-Tuderman, L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521.
- Halfter, W.; Dong, S.; Schurer, B.; Cole, G.J. Collagen XVIII is a basement membrane heparin sulfate proteoglycan. J. Biol. Chem. 1998, 273, 25404–25412.
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55.
- Pastor-Pareja, J.C. Atypical basement membranes and basement membrane diversity–what is normal anyway? J. Cell Sci. 2020, 133, jcs241794.
- Kvist, A.J.; Nyström, A.; Hultenby, K.; Sasaki, T.; Talts, J.F.; Aspberg, A. The major basement membrane components localize to the chondrocyte pericellular matrix–a cartilage basement membrane equivalent? Matrix Biol. 2008, 27, 22–33.
- Hagedorn, M.; Esser, P.; Wiedemann, P.; Heimann, K. Tenascin and decorin in epiretinal membranes of proliferative vitreoretinopathy and proliferative diabetic retinopathy. Germ. J. Ophthalmol. 1993, 2, 28–31.
- Immonen, I.; Tervo, K.; Virtanen, I.; Laatikainen, L.; Tervo, T. Immunohistochemical demonstration of cellular fibronectin and tenascin in human epiretinal membranes. Acta Ophthalmol. 1991, 69, 466–471.
- Pattwell, D.M.; Sheridan, C.M.; Le Goff, M.; Bishop, P.N.; Hiscott, P. Localisation of opticin in human proliferative retinal disease. Exp. Eye Res. 2010, 90, 461–464.
- Alexander, R.A.; Hiscott, P.; McGalliard, J.; Grierson, I. Oxytalan fibres in proliferative vitreoretinopathy. Germ. J. Ophthalmol. 1992, 1, 382–387.
- Weller, M.; Wiedemann, P.; Bresgen, M.; Heimann, K. Vitronectin and proliferative intraocular disorders. I. A colocalisation study of the serum spreading factor, vitronectin and fibronectin in traction membranes from patients with proliferative vitreoretinopathy. Int. Ophthalmol. 1991, 15, 93–101.
- Hiscott, P.; Larkin, G.; Robey, H.L.; Orr, G.; Grierson, I. Thrombospondin as a component of the extracellular matrix of epiretinal membranes: Comparisons with cellular fibronectin. Eye 1992, 6, 566–569.
- Casaroli Marano, R.P.; Vilaró, S. The role of fibronectin, laminin, vitronectin and their receptors on cellular adhesion in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2791–2803.
- Grisanti, S.; Guidry, C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Investig. Ophthalmol. Vis. Sci. 1995, 36, 391–405.
- Bornstein, P. Matricellular proteins: An overview. J. Cell Commun. Signal. 2009, 3, 163–165.
- Weller, M.; Esser, P.; Bresgen, M.; Heimann, K.; Wiedemann, P. Thrombospondin: A new attachment protein in preretinal traction membranes. Eur. J. Ophthalmol. 1992, 2, 10–14.
- Hiscott, P.; Hagan, S.; Heathcote, L.; Sheridan, C.M.; Gronewald, C.P.; Grierson, I.; Wong, D.; Paraoan, L. Pathobiology of epiretinal and subretinal membranes: Possible roles for the matricellular proteins thrombospondin 1 and osteonectin (SPARC). Eye 2002, 16, 393–403.
- Yoshida, S.; Nakama, T.; Oshokawa, K.; Kakao, S.; Sonoda, K.; Ishibashi, T. Periostin in vitreoretinal diseases. Cell Mol. Life Sci. 2017, 74, 4329–4337.
- Russel, S.R.; Hageman, G.S. Chondroitin sulfate-induced generation of epiretinal membranes. Arch. Ophthalmol. 1992, 110, 1000–1006.
More
